2. State Key Laboratory of Clean Energy Utilization, Zhejiang University, Hangzhou 310027, China
2. 浙江大学 能源清洁利用国家重点实验室, 浙江 杭州 310027
Nitrogen oxides (NOx) emission from fossil fuel combustion is one of the most important environmental problems because it contributes to the formation of acid rain, photochemical smog and ozone depletion. Oxidizing NO into NO2 by O2 is an essential pre-requisite step for NOx removal as NO2 promotes the selective ca-talytic reduction and enhances the absorption of NO in wet flue gas desulfurization system[1]. One of the effective methods to increase the proportion of NO2 in exhaust gas is passing gas over a strong oxidation catalyst.
Transition metal oxides, supported noble metal catalyst, active carbon and zeolite are the primary catalysts for NO catalytic oxidation reaction[1-4]. Among these catalysts, transition metal oxides have been stu-died widely due to its excellent redox ability and the moderate cost. Many studies reported that cerium oxide has strong ability for oxygen storage and shows a good performance in oxidation reactions[5]. Manganese oxides present high low-temperature catalytic activity and shows strong ability to interact with cerium oxide which leads to the formation of solid-solution structure, the promotion of the activity of lattice oxygen, and the enhancement of catalytic activity[6]. Therefore, the manganese-cerium composite catalysts have been stu-died extensively in treatment of liquid and gaseous pollutants due to their superior catalytic performance[5, 7-9].
However, compared with the commercially noble metal supported catalyst, the catalytic activity for NO catalytic oxidation of the manganese-ceriumcomposite oxides catalyst under current preparation method is still lower, which limits it to be the substitute of commercial catalyst. Therefore, it is of great significance to carry on research on how to enhance the catalytic oxidation ability of this transition metal oxide catalyst. In recent years, non-thermal plasma has been applied in the preparation of catalysts as plasma-assisted preparation can change catalyst properties and improve cataly-tic activity. Non-thermal plasma contains a large number of active species, including excited particles, electrons and ions, with a macroscopic temperature of 25~200 ℃. These active species can help decompose nitrates and organics in precursor of the catalyst and lead to the formation of surface defects and active sites[10-13]. Thus pretreatment of the catalyst using plasma shows a promising method to improve the redox ability and catalytic performance of the manganese-cerium mixed-oxides catalyst[12, 14-16]. In this work, MnCeOx catalysts were prepared using the plasma-assisted sol-gel method and tested in the oxidation of NO. The effects of plasma in different discharge atmosphere including Ar, N2 and air were compared. The physical and chemical properties of the catalysts were characterized.
1 Experimental 1.1 Catalyst preparationMnCeOx precursor with a Mn: Ce atom ratio of 3: 1 was prepared by the sol-gel method[15]. The sol was heated at 80 ℃ till gel was formed. The sample was dried at 110 ℃ overnight. This sample was denoted as MnCeOx w/o P or C. Then the sample divided into two portions. One portion calcined in air at 300 ℃. The obtained sample was denoted as MnCeOx-C. The other portion of the sample was put into the discharging tube and treated by plasma for 90 min, followed by the calcination in air at 300 ℃. The plasma was generated by high-frequency dielectric barrier discharge with discharge power of 180 W. This sample was nominated as MnCeOx-M (M was noted as discharge atmosphere, Ar, N2 or Air).
1.2 Catalytic activity testsExperiments of NO catalytic oxidation were carried out at atmospheric pressure in the temperature range of 100~350 ℃ in a cylindrical fixed-bed quartz reactor (diameter = 8 mm) containing 0.2 g catalyst of 60~80 mesh. The reactor was connected to Testo 350XL gas analyser for NO and NO2 measurement. The gas flow and gas hourly space velocity (GHSV) in all experiments were 1 200 mL/min and 360 000 mL-1g-1h-1; The simulated gas mixtures used contained 400 ppm NO, 5% O2 and balance N2. The NO oxidation efficiency (X) and the rate constant (k) were calculated as follows:
| $ X = \frac{{{{\left[{NO} \right]}_{in}} - {{\left[{NO} \right]}_{out}}}}{{{{\left[{NO} \right]}_{in}}}} \times 100\% $ | (1) |
| $ k = \frac{F}{W}\ln \left( {1 - X} \right) $ | (2) |
Where [NO]in and [NO]out represented the inlet and outlet concentrations of gaseous NO, respectively; F was the total flow rate and W was the weight of the catalyst.
1.3 Catalyst characterizationThermogravimetric analysis(TGA) was performed by using a TA Q500 TGA instrument. The samples (3~4 mg) were heated from 50 to 800 ℃ in nitrogen at a heating rate of 10 ℃/min.
Fourier-transform infrared spectroscopy (FTIR) was carried out to investigate the functional groups species using a Nicolet 5700 spectrometer. The spectrum was generated and collected 32 times and the background line obtained was automatically subtracted. The spectra were recorded from 4 000~400 cm-1.
The BET surface area and pore size distribution were obtained by measuring N2 adsorption and desorption at liquid nitrogen temperature (77 K) with Micromeritics ASAP 2020 system. Prior to N2 adsorption, the samples were degassed at 250 ℃ for 3 h.
The powder X-ray diffraction (XRD) was carried out on a Rigaku D/max-rA system using monochromated Cu Kα radiation (40 kV, 200 mA) at a scan rate of 4°/min with a 0.0167° step size in the 2θ range between 10° and 90°.
Temperature programmed reduction (TPR) of hydrogen was performed on a custom-made TCD setup using 100 mg catalysts to measure the redox properties. Prior to each measurement, the samples were pretreated under flowing pure Ar at 200 ℃ for 30 min, and this was followed by cooling to 50 ℃ in atmosphere of Ar containing 10% H2 at a flow rate of 30 mL/min. TPR runs were carried out from 50~700 ℃ with linear heating rate (10 ℃/min) in the same atmosphere.
The X-ray photoelectron spectra (XPS) measurement of O 1s, Mn 2p, and Ce 3d regions was performed a Thermo ESCALAB 250 electron spectrometer equipped with an Al Kα X-rays (hν=1 486.6 eV) and a hemispherical electron analyzer being operated at a constant pass energy (20 eV). Sample charging effects were eliminated by correcting the observed spectra with a C 1s binding energy (BE) value of 284.6 eV.
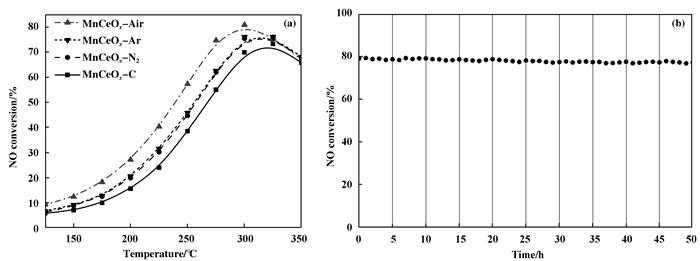
2 Results and Discussion 2.1 Catalytic activitiesFig. 1(a) shows the NO oxidation efficiency of the manganese-cerium oxides catalysts prepared by plasma pretreatment and traditional method. The oxidation efficiency increases as the temperature increases until reaches the equilibrium of the reaction. The order of oxidation efficiency is as follows: MnCeOx-Air > MnCeOx-Ar ≈ MnCeOx-N2 > MnCeOx-C. Compared with the catalyst prepared by traditional method, the catalytic activities are improved by the pretreatment of plasma, especially at 250~275 ℃. The NO conversion of MnCeOx-Air is 75% at 275 ℃, which is 20% higher than that of MnCeOx-C. In Fig. 1(b), it can be seen that the MnCeOx-Air catalyst has a favorable stability within 50 h.
|
Figure 1 (a) Catalytic activity of the catalysts along with temperatures; (b) Catalytic performance of the MnCeOx-Air catalyst within 50 h |
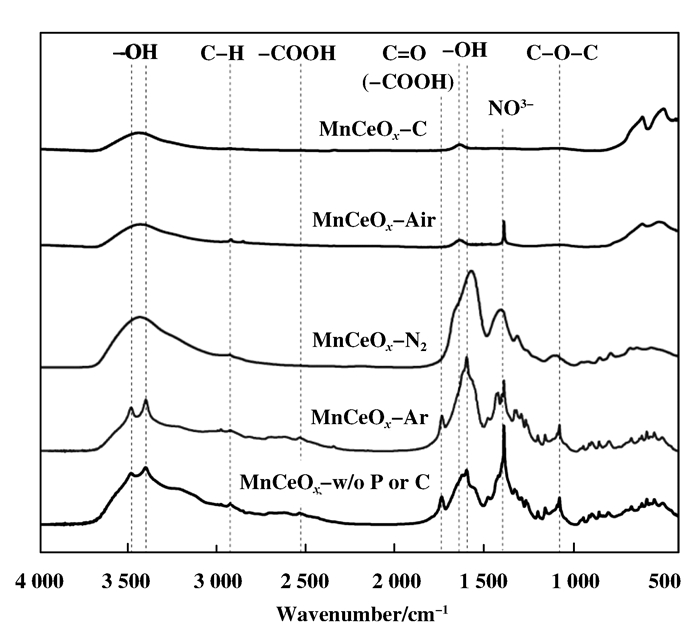
To study the characters and components at the surface of the precursor treated by plasma in different discharge atmosphere, FTIR was utilized to analyze these samples. The results are displayed in Fig. 2. The surface of the precursor without treatment has numerous groups including —COOH, —NO3-, —OH, C—O—C and C = O. The peak at 1 388 cm-1 is corresponding to the nitrates. The peak is weakened by Ar plasma treatment, which demonstrates that Ar plasma could decompose partial nitrates. The peak at 1 734 cm-1 is contributed to the C = O in —COOH, and the peak at 1 073 cm-1 is contributed to C—O—C in polyethylene glycol (PEG). No obvious change for these two peaks is observed after Ar plasma treatment, which demonstrates that Ar plasma cannot decompose organics. This is because the active species in Ar plasma is only metastable Ar atoms[17], which have weak reaction with the precursor.
|
Figure 2 The FTIR spectra of the catalyst precursors before calcination |
After N2 plasma treatment, the —COOH peak vanishes and both the C—O—C and —NO3- peaks weaken notably. It demonstrates that N2 plasma treatment can decompose all carboxyl group and partial polyethylene glycol and nitrates, which results from the reactions between the precursor and excited or metastable N and N2 in N2 plasma, which is more active than metastable Ar.
The infrared spectroscopy of the sample treated by air plasmas is almost the same with that of the sample after calcination, except trace amount of residue nitrates. As our experimental experience, if catalyst were placed in the plasma discharge zone, NO and NO2 could be oxidized by O2 in air, and then adsorbed by the catalyst. Therefore the residue nitrates maybe caused by the decomposition-adsorption equilibrium. It indicates that air plasmas could decompose nitrates and organic matters almost completely and gain pure oxides with trace nitrates. That is because the oxidative active components including O, O3 formed in air plasma can decompose nitrates and organics in the precursor of catalyst efficiently.
TGA
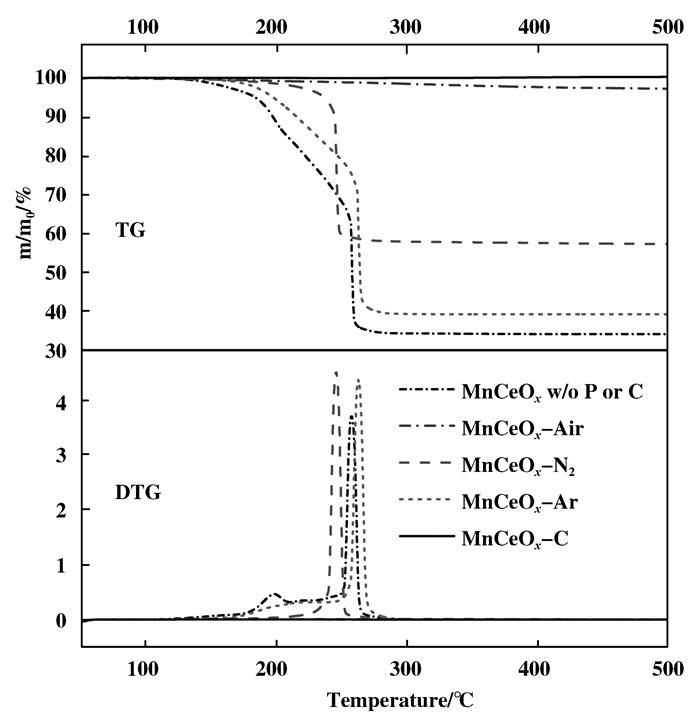
The results of TGA for the samples treated by plasma but without calcinations are displayed in Fig. 3. Compare with the result of FTIR, it is found that the weight loss before the temperature of 230 ℃ is attributed to the desorption of absorbed water, and the decomposition of carboxyl group and nitrates to intermediates, such as Mn(OH)2. The precursor without any treatment shows an obvious weight loss about 11% at 200 ℃. Its weight loss at 250~270 ℃ could be contributed to the decomposition of the intermediates and the organics to Mn2O3. Then the weight does not change as the temperature continues rising. The whole weight loss is about 66% eventually.
|
Figure 3 TG and DTG curves of the catalyst precursors before calcination |
The sample prepared by Ar plasma shows a whole weight loss of 61.6%, which was just 4.4% lower than the precursor without plasma treatment. This is assistance with the result of FTIR, that Ar plasma could only decompose partial nitrates. The sample treated by N2 plasma shows a weight loss of about 42.5%. The peak at 200 ℃ disappears, and the weight loss for organics oxidation moves to a lower temperature of 230 to 252 ℃. Because the N2 plasma decomposes the organics to some matters which are easy to be oxidized, the weight loss of the sample treated by N2 plasma shifts to a lower temperature. The sample treated by air plasma shows weight loss about 4.5% and no noticeable weight loss peak is observed. It indicates that the nitrates and organics have been decomposed almost completely in the treatment process by air plasma. Therefore the pretreatment of air plasma help avoid sintering and agglomeration occurring at high temperature in calcination.
2.3 Characteristics of the catalystsBET
Table 1 gives the specific surface area, pore volume and pore size of the catalysts prepared by plasma pretreatment and traditional calcination method. The catalysts pretreated by N2 and Ar plasma shows little difference in specific surface area, pore volume and pore size with the sample prepared by traditional met-hod, while the sample treated by air plasma shows much larger values of all the parameters. The specific surface area of MnCeOx-Air is 165 m2g-1, which is 2.4 times larger than that of MnCeOx-C.
| Table 1 The physical characteristics of the catalysts |
XRD
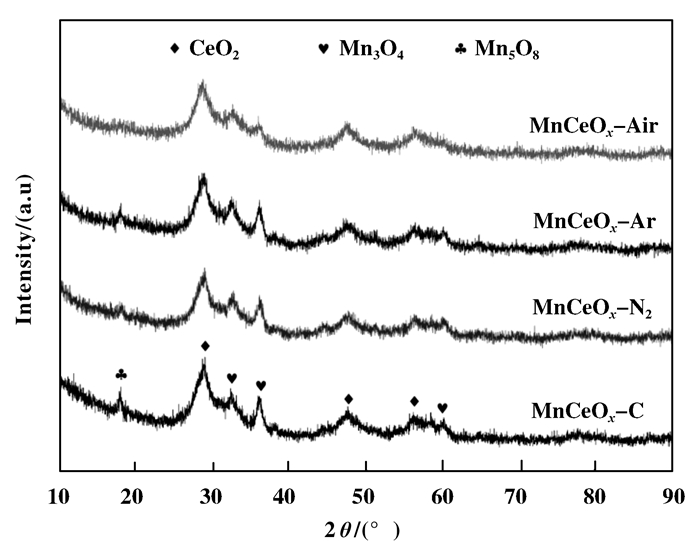
The XRD patterns of the samples prepared by plasma pretreatment and traditional method are dis-played in Fig. 4. As shown in Fig. 4, in the pattern of MnCeOx-C, the peaks of CeO2, Mn3O4 and Mn5O8 can be found. The CeO2 peaks of the samples treated by plasmas are weaker than that of MnCeOx-C, which suggests that plasma treatment promotes the dispersion of CeO2 component. The Mn5O8 peaks of MnCeOx-N2 and MnCeOx-Ar are much weaker than that of MnCeOx-C, while the Mn5O8 peak of MnCeOx-Air vanishes. It demonstrates that plasma pretreatment enhance the interaction of MnOx and CeO2, and more solid solution structure is formed. The peak of CeO2, Mn3O4 and Mn5O8 in MnCeOx-Air is weaker than that of other samples, which demonstrates the solid solution structure of MnCeOx is well formed in MnCeOx-Air. As reported in the research of MnOx-CeO2 mixed oxides, the solid solution has higher oxidation efficiency than MnOx and CeO2[18-19], which is in the good agreement with the results of this study.
|
Figure 4 XRD patterns of the catalysts |
XPS
The formation of solid solution structure can change the valence of Mn and Ce in mixed-oxides and increase the amount of active oxygen. In order to analyze the valence states of the metal element and the type of oxygen on the surface of the studied samples, XPS spectra of O 1s and Ce 2p were measured, and the results are shown in Fig. 5 and Table 2. Table 2 shows the percentage of oxygen on catalyst surface. It is found that the catalysts surface gets relatively rich in O value with the pretreatment of plasma. The spectra in Fig. 5 are deconvolved for the convenience of observation. The oxygen species on catalyst surface plays a significant role in catalytic oxidation. The peak at 529 eV is contributed to lattice oxygen (noted as Oβ) in oxides, while the peak at 531 eV is attributed to the surface adsorbed oxygen (noted as Oα), such as the chemical absorbed oxygen and the hydroxyl-like group at catalyst surface[20]. The Oα which is more active than Oβ is the major species for oxidation reaction and has significant effect on catalytic activity[21]. As shown in Fig. 5 and Table 2, the samples pretreated by plasma present higher Oα/(Oα+Oβ) values than the sample prepared by traditional method. Quantitatively, it was found that the Oα/(Oα+Oβ) value of MnCeOx-Air, MnCeOx-Ar and MnCeOx-N2 is 14.0%, 2.2% and 3.6% higher than that of MnCeOx-C (26.2%), respectively, which suggests that plasma pretreatment can notably increase the oxygen vacancies and surface active oxygen, which is beneficial for catalytic oxidation.
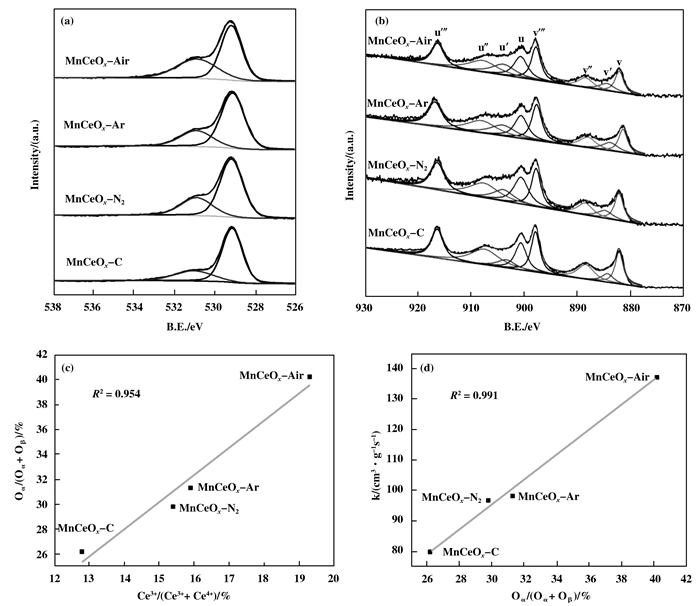
|
Figure 5 XPS spectra for (a) O 1s and (b)Ce 3d. (c) Correlations between the relative concentrations of surface-adsorbed oxygen Oα/(Oα+Oβ) and the relative concentrations of Ce3+/(Ce3++Ce4+). (d) Correlations between the relative concentrations of surface-adsorbed oxygen Oα/(Oα+Oβ) and the rate constant (k) at 275 ℃ |
| Table 2 XPS results of the catalysts |
In the Ce 3d spectra, the U, U", U''', V, V" and V''' peaks are contributed to Ce4+, while the U' and V' peaks are attributed to Ce3+. Table 2 summarizes the calculated values of quantitative analysis. MnCeOx-Air has the highest proportion of Ce3+, which is 6.5% higher than that of MnCeOx-C. And the proportion of Ce3+ in MnCeOx-Ar and MnCeOx-N2 is 3.1% and 2.6% higher than that of MnCeOx-C, respectively. The existence of Ce3+ is important to the formation of the surface active oxygen on catalyst surface, because Ce3+ can lead to charge imbalance, oxygen vacancies and unsaturated chemical bonds[22]. Due to more solid solution is formed by plasma pretreatment, as discussed in XRD, the portion of the relative amount of Ce3+/(Ce3++Ce4+) is increased. As a result, oxygen vacancies and surface active oxygen in plasma-assisted prepared catalysts increase, which is confirmed by the results of relative concentration ratios of Oα/(Oα+Oβ). As presented in Fig. 5(c), good correlation can be observed between the relative concentration ratios of Ce3+/(Ce3++Ce4+) and relative concentration ratios of Oα/(Oα+Oβ), indicating that the relative concentration ratios of surface adsorbed oxygen are directly rela-ted to the concentration of Ce3+ on catalyst surface. Therefore, the well-formed solid solution results in the increase of relative amount of Ce3+ and the increase of oxygen vacancies and surface active oxygen, which has higher mobility than lattice oxygen and contributes to the enhanced catalytic performance. As shown in Fig. 5(d), the rate constant (k) has a linear correlation with relative concentration ratios of Oα/(Oα+Oβ), indicating the catalytic performance is sufficiently related to the relative concentration ratios of surface adsorbed oxygen.
H2-TPR
Fig. 6 shows the H2-TPR profiles of the studied catalysts. Profile of the catalysts showed two reduction peaks. One locates in low temperature between 200 to 250 ℃, which is attributed to the reduction of Mn4+ to Mn3+, and the other in high temperature between 350 to 400 ℃, is attributed to the reduction of Mn3+ to Mn2+. The catalysts pretreated by plasmas displays stronger peaks in low temperature and weaker peaks in high temperature compared with the catalyst prepared by traditional method, which indicates that plasma pretreatment can improve oxygen mobility and oxidation-reduction ability at low temperature[23]. As discussed above in XPS results, there is more oxygen species and higher portion of surface adsorbed oxygen on plasma pretreated catalysts, which is easier to be reduced by H2 and has an important influence on catalytic oxidation[24].
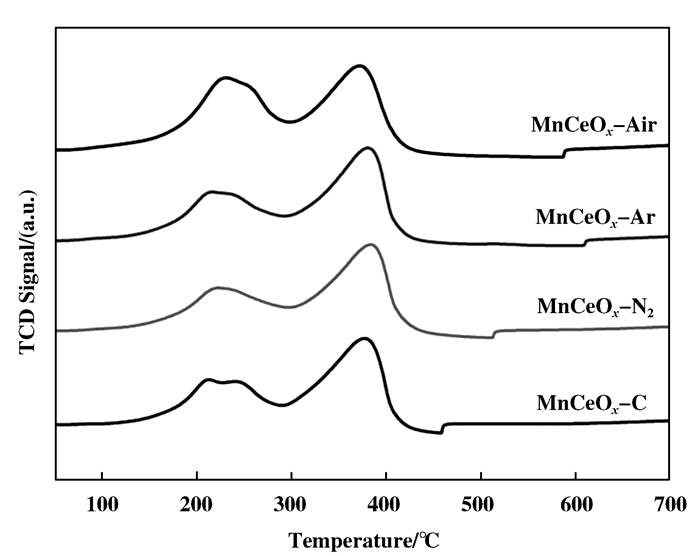
|
Figure 6 H2-TPR profiles of the catalysts |
In conclusion, plasma treatment can form more Mn-Ce solid solution structures and enhance the speci-fic surface area of the catalysts and the Ce3+ component at catalyst surface, all of which can notably increase the oxygen vacancies and surface active oxygen. The increase of surface oxygen species enhances the redox performance of catalyst leading to the improvement of catalytic activity. Besides, the air plasma pretreatment shows the largest influence on the physical and chemical properties of the catalyst. The influence of the N2 or Ar plasma treatment on catalyst is similar, and both of them are weaker than that of air plasma. That is because the oxidative active components including O, O3 formed in air plasma can decompose nitrates and orga-nics in the precursor of catalyst efficiently and lead to the formation of oxides before calcination. However, there are only some metastable Ar atoms in Ar plasma and some excited or metastable N and N2 active components in N2 plasma. These active components have weak reaction with the precursor of the catalyst, so have a weak influence on the catalytic performance.
3 ConclusionPlasma pretreatment can notably enhance the catalytic oxidation activity of MnCeOx catalyst. The NO conversion efficiency of MnCeOx-Air is 20% higher than that of MnCeOx-C. Air plasma treatment has the largest influence on the physical and chemical properties, while the influences of N2 and Ar plasmas treatments are similar and both of them were weaker than air plasma. Plasma pretreatment, especially in air discharge atmosphere, enhances the formation of solid solution between MnOx and CeO2, which results in hig-her portion of Ce3+, more surface active oxygen and the improvement of redox ability of the catalysts. All of them are the major reasons for the improvement of catalytic performance.
| [1] |
a. Wang Q, Park S, Duan L, et al. Activity, stability and characterization of NO oxidation catalyst Co/KxTi2O5[J]. Appl Catal B, 2008,85(1):10-16. b. Liu Lu(刘璐), Zheng Cheng-hang(郑成航), Gao Xiang(高翔). Density functional theory study on the adsorption of NO on Mn2O3 (110) surface(NO在Mn2O3 (110)表面吸附的密度泛函理论研究)[J]. J Mol Catal(China)(分子催化), 2017,31(6):544-552. c. Jin Qi-jie(金奇杰), Sui Guo-rong(眭国荣), Liu Qing(刘青), et al. Compatibility optimization of Mn-Mo-W-Ox catalyst for selective catalytic reduction of NO by NH3(Mn-Mo-W-Ox脱硝催化剂活性组分的配伍优化)[J]. J Mol Catal(China)(分子催化), 2017,31(2):159-168. d. Su Qian(苏潜), Huang Yan(黄妍), Zhang Yin(张颖), et al. Effects of copper sources on selective catalytic reduction of NO with NH3 of Cu-SAPO-34(铜源对Cu-SAPO-34氨催化还原NO性能的影响)[J]. J Mol Catal(China)(分子催化), 2016,30(2):151-158. |
| [2] |
a. Rankovic N, Nicolle A, Berthout D, et al. Extension of a kinetic model for NO oxidation and NO storage to fixed-bed Pt/Ba/AlO catalysts[J]. Catal Commun, 2010,12(1):54-57. b. Yu Rui(喻瑞), Zhao Zhen-chao(赵侦超), Zhang Zhen-yu(张振宇), et al. Effects of Pt modification and reaction atmosphere on the catalytic performances of Cu/Al-rich beta zeolite in selective catalytic reduction of NO by NH3(Pt修饰和反应气氛对富铝Cu-Beta分子筛NH3-SCR反应性能的影响)[J]. J Mol Catal(China)(分子催化), 2016,30(5):428-434. c. Guo Zhi-min(郭志敏), Yuan Jian(袁坚), Peng Xiao-jin(彭小晋), et al. The effect of sodium salts on catalytic activity of V2O5-WO3/TiO2 over NH3-SCR reaction (钠盐对V2O5 -WO3/TiO2催化剂NH3-SCR反应活性的影响)[J]. J Mol Catal(China)(分子催化), 2016,30(6):547-556. |
| [3] | Sousa J P S, Pereira M F R, Figueiredo J L. Catalytic oxidation of NO to NO2 on N-doped activated carbons[J]. Catal Today, 2011, 176(1): 383–387. DOI:10.1016/j.cattod.2010.11.040 |
| [4] | Metkar P S, Balakotaiah V, Harold M P. Experimental and kinetic modeling study of NO oxidation:Comparison of Fe and Cu-zeolite catalysts[J]. Catal Today, 2012, 184(1): 115–128. DOI:10.1016/j.cattod.2011.11.032 |
| [5] | Xie Xing-xing(谢兴星), Fei Zhao-yang(费兆阳), DaiI Yong(戴勇), et al. Structure of ceria-based mixed oxides and its influence on HCl catalytic oxidation performance(铈基复合氧化物的结构及其对HCl催化氧化性能的影响)[J]. J Mol Catal (China)(分子催化), 2014, 28(6): 507–514. |
| [6] | Wiatros-Motyka M M, Sun C G, Stevens L A, et al. Experimental study of mercury removal from exhaust gases[J]. Fuel, 2013, 109(28): 451–457. |
| [7] | Lin Jun-min(林俊敏), Fu Ming-li(付名利), Zhu Wen-bo(朱文波), et al. An investigation of surface reactive species on MnOx(0.4)-CeO2 catalyst towards soot oxidation(氧化碳烟的MnOx(0.4)-CeO2催化剂表面活性物种研究)[J]. J Mol Catal (China)(分子催化), 2014, 28(2): 165–173. |
| [8] | Lin F, Wu X, Liu S, et al. Preparation of MnOx-CeO2-Al2O3 mixed oxides for NOx-assisted soot oxidation:Activity, structure and thermal stability[J]. Chem Eng J, 2013, 226(24): 105–112. |
| [9] | Li X, Zhang S, Jia Y, et al. Selective catalytic oxidation of NO with O2 over Ce-doped MnO/TiO2 catalysts[J]. J Nat Gas Chem, 2012, 21(1): 17–24. DOI:10.1016/S1003-9953(11)60327-7 |
| [10] | Nakamura I, Negishi N, Kutsuna S, et al. Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light activity for NO removal[J]. J Mol Catal A, 2000, 161(1/2): 205–212. |
| [11] | Li Z H, Tian S X, Wang H T, et al. Plasma treatment of Ni catalyst via a corona discharge[J]. J Mol Catal A, 2004, 211(1): 149–153. |
| [12] | Liu Y, Pan Y X, Kuai P Y, et al. Template removal from ZSM-5 zeolite using dielectric-barrier discharge plasma[J]. Catal Lett, 2010, 135(3/4): 241–245. |
| [13] | Zou J J, Zhang Y P, Liu C J, et al. Reduction of supported noble-metal ions using glow discharge plasma[J]. Langmuir, 2006, 22(26): 11388–11394. DOI:10.1021/la061795b |
| [14] | Liu Y, Pan Y X, Wang Z J, et al. Facile and fast template removal from mesoporous MCM-41 molecular sieve using dielectric-barrier discharge plasma[J]. Catal Commun, 2010, 11(6): 551–554. DOI:10.1016/j.catcom.2009.12.017 |
| [15] | Liu L, Zheng C, Wu S, et al. Manganese-cerium oxide catalysts prepared by non-thermal plasma for NO oxidation:Effect of O2 in discharge atmosphere[J]. Appl Surf Sci, 2017, 416: 78–85. DOI:10.1016/j.apsusc.2017.04.020 |
| [16] | Kuai P Y, Liu C J, Huo P P. Characterization of CuO-ZnO catalyst prepared by decomposition of carbonates using dielectric-barrier discharge plasma[J]. Catal Lett, 2009, 129(3/4): 493–498. |
| [17] | Fateev A, Leipold F, Kusano Y, et al. Plasma chemistry in an atmospheric pressure Ar/NH3 dielectric barrier discharge[J]. Plasma Process Polym, 2005, 2005(3): 193–200. |
| [18] | Machida M, Uto M, Kurogi D, et al. MnOx-CeO2 binary oxides for catalytic NOx sorption at low temperatures sorptive removal of NOx[J]. Chem Mater, 2000, 12(10): 3158–3164. DOI:10.1021/cm000207r |
| [19] | Tikhomirov K, Krocher O, Elsener M, et al. MnOx-CeO2 mixed oxides for the low-temperature oxidation of diesel soot[J]. Appl Catal B, 2006, 64(1): 72–78. |
| [20] | Dupin J C, Gonbeau D, Vinatier P, et al. Systematic XPS studies of metal oxides, hydroxides and peroxides[J]. Phys Chem Chem Phys, 2000, 2(6): 1319–1324. DOI:10.1039/a908800h |
| [21] | Chen H, Sayari A, Adnot A, et al. Composition-activity effects of Mn-Ce-O composites on phenol catalytic wet oxidation[J]. Appl Catal B, 2001, 32(3): 195–204. DOI:10.1016/S0926-3373(01)00136-9 |
| [22] | Chen L, Li J H, Ablikim W, et al. CeO-WO mixed oxides for the selective catalytic reduction of NO over a wide temperature range[J]. Catal Lett, 2011, 141(12): 1859–1864. DOI:10.1007/s10562-011-0701-4 |
| [23] | Liu L, Gao X, Song H, et al. Study of the promotion effect of iron on supported manganese catalysts for NO oxidation[J]. Aerosol Air Qual Res, 2014, 14(3): 1038–1046. |
| [24] | Liao Y, Fu M, Chen L, et al. Catalytic oxidation of toluene over nanorod-structured Mn-Ce mixed oxides[J]. Catal Today, 2013, 216(6): 220–228. |
 2018, Vol. 32
2018, Vol. 32


