2. 兰州理工大学 石油化工学院, 甘肃 兰州 730050;
3. 河南金马能源股份有限公司, 河南 济源 455000
2. School of petroleum and chemical engineering, Lanzhou University of Technology, Lanzhou 730050, China;
3. Henan Jinma Energy Co., Ltd., Jiyuan 455000, China
随着我国工业及城市的发展, 氮氧化物(NOx)的排放逐渐增加, 进一步导致了大气环境污染的加剧[1-5].随着我国对大气污染防控治理的重视及人类日益增强的环保意识, 越来越多的科研人员开始研究NOx的控制和消除方法. NOx的控制及消除方法主要有以下几种:预燃烧、后燃烧技术及燃烧改造.目前应用较为广泛的则是后燃烧控制技术, 以此来实现NOx的有效消除.
消除NOx的方法包括SCR和SNCR等方法, 其中SCR技术现已得到了非常广泛的应用[6-8].该技术主要是利用催化剂来催化NOx与NH3之间的反应, 其具有高效的脱硝效率、优良的选择性、可靠的耐久性及较高的重复利用性等[9].随着国内外对于SCR脱硝技术的深入研究, SCR反应系统中如何降低催化剂的费用, 同时保证高效的催化效果成为了一个重要的研究课题[10].
| $ 4{\rm{NO}} + 4{\rm{N}}{{\rm{H}}_3} + {{\rm{O}}_2} \to 4{{\rm{N}}_2} + 6{{\rm{H}}_2}{\rm{O}} $ | (1) |
| $ 2{\rm{N}}{{\rm{O}}_2} + 4{\rm{N}}{{\rm{H}}_3} + {{\rm{O}}_2} \to 3{{\rm{N}}_2} + 6{{\rm{H}}_2}{\rm{O}} $ | (2) |
| $ 6{\rm{NO}} + 4{\rm{N}}{{\rm{H}}_3} \to 5{{\rm{N}}_2} + 6{{\rm{H}}_2}{\rm{O}} $ | (3) |
| $ 6{\rm{N}}{{\rm{O}}_2} + 8{\rm{N}}{{\rm{H}}_3} \to 7{{\rm{N}}_2} + 12{{\rm{H}}_2}{\rm{O}} $ | (4) |
首先, 将NH3喷入烟气中, NH3在随烟气的运动中与催化剂相遇, 被催化剂的活性点位所吸附; 其次, 烟气中所含的NOx则与吸附在活性点位上的NH3发生反应[11], N2及H2O作为反应产物, 则从催化剂上脱附[2], 而同时活性位点被释放; 最后, 烟气中所含的O2则扩散并吸附在催化剂的活性位点上, 并进而置换出氢, 最终使催化剂的活性得以恢复和保持[2, 13].
关于SCR的反应机理, 经过长期研究认为, E-R(Eley-Rideal)和L-H(Lamgmuir-Hinshelwood)是SCR所遵循的两种主要反应机理[15].如图 1所示, 对于E-R机理, 其核心和关键是NH3向活跃的NH2转化.被吸附的NH2或者NH3会与气态NO发生反应生成活性中间体NH2NO/NH3NO, 最后被分解为N2和H2O.同时高价氧化还原位点将会被还原为低价位点, 随后被O2再氧化进而完成氧化还原圈[16].而对于L-H机理, NH3吸附在酸性位点上形成NH4+, NO被高价氧化还原位点氧化成具有活性的双齿硝酸盐/桥式硝酸盐/单齿硝酸盐, 随后与NH4+反应形成NH4NO2、NH4NO3, 最后被分解为N2和H2O.同时高价氧化还原位点将会被还原为低价位点, 随后被O2再氧化进而完成氧化还原圈[16].
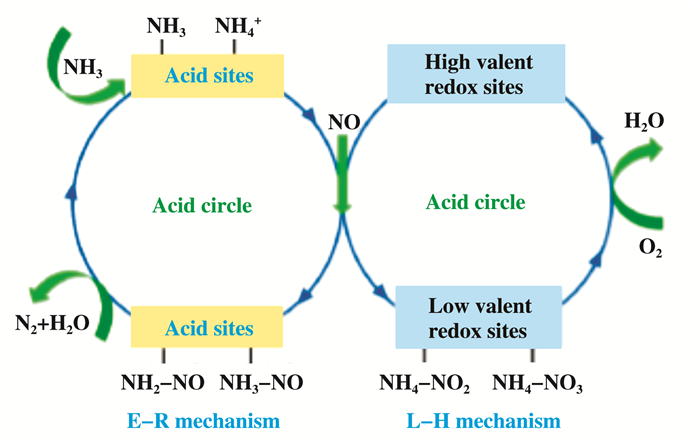
|
图 1 SCR反应路径示意图 Fig.1 Schematic diagram of SCR reaction path |
许多金属催化剂的SCR反应在低温下遵循L-H机理, 而在高温下遵循E-R机理[17-18].例如: Li等[19]通过研究MoO3-CeAlOx催化剂发现, 在300 ℃的反应温度下, MoO3-CeAlOx催化剂中被吸附的NH4+与NH3可以与吸附的单齿硝酸盐和双齿硝酸盐(L-H)机制反应, 在300 ℃的反应温度以上, NH3氧化脱氢所衍生出的NH2中间体和被保留吸附的NH3可以与气态NO发生反应生成N2和H2O(E-R机制).如图 2所示, 兰等[20]通过实验证明, Mn-Fe尖晶石类催化剂的催化反应属于L-H机理和E-R机理的结合.基于L-H反应, NO3-和NH3反应, 生成NH, NH则与NO反应, 生成N2O.基于E-R反应, 气态NO浓度的增加会抑制NH的形成, 进而减少N2O的生成[21].同时, NH3分子也可以裂解生成聚集吸附的N, 并与气态NO反应生成N2O[22].
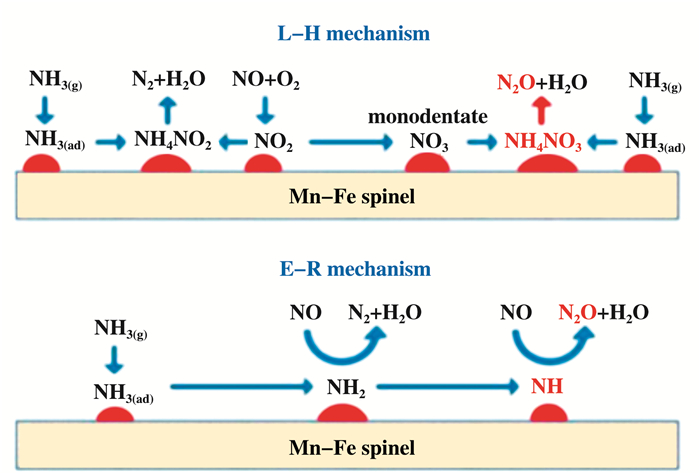
|
图 2 Mn-Fe尖晶石催化剂SCR反应路径 Fig.2 SCR reaction path over Mn-Fe spinel catalyst |
对于脱硝催化剂, 制备方法同样会影响其催化效果[2, 23-24]. Liu等[25-26]研究了制备方法对Fe2O3-TiO2催化剂催化活性的影响, 表征测试结果表明, 共沉淀法制备的催化剂的催化活性最高.在5000 h-1空速下和350~450 ℃的温度区间内, 催化效率大于90%, 原因是共沉淀法制备出的催化剂含有更多的Fe-O-Ti活性物种.
Qi等[27]系统的研究了制备方法对SCR反应速率的影响, 结果表明, 柠檬酸法(CA)制备的CeO2-MnOx催化剂的催化活性最高.表征结果显示, 在CA法制备的催化剂(CeO2-MnOx)中, 部分Mn进入了CeO2晶格, 表面氧空位增多, 使得活性中间产物NH2在SCR反应过程中更容易产生[28], 这是催化活性增强的主要原因.
如图 3及图 4所示, Gao等[29]的研究结果则表明, 凝胶溶胶法所制备的CeTi催化剂, 不但具有最强的催化活性, 同时还具有极高的抗硫性[30].表征分析结果表明, 相对于其他催化剂, SG法制备的催化剂上所吸附的SO2被抑制, 其原因主要是铈钛之间的相互作用和不同价态的铈之间的相互作用.这也是凝胶溶胶法所制备的CeTi催化剂抗硫性高的原因.其通过表征分析发现, 催化剂的氧化还原性、比表面积、铈钛的存在状态和相互作用的强度都会影响SCR反应.综上, 制备方法也会影响催化剂自身的结构及活性[31].
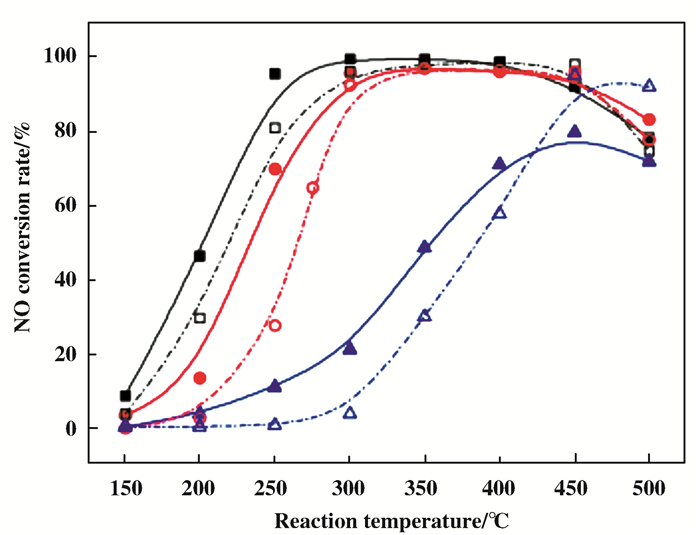
|
图 3 制备方法对催化剂活性的影响 Fig.3 Effect of preparation method on catalyst activity ■ □溶胶凝胶法; ● ○浸渍法; ▲ △共沉淀法 ■ □ Sol-gel method; ● ○ Impregnation method; ▲ △ Co-precipitation method |
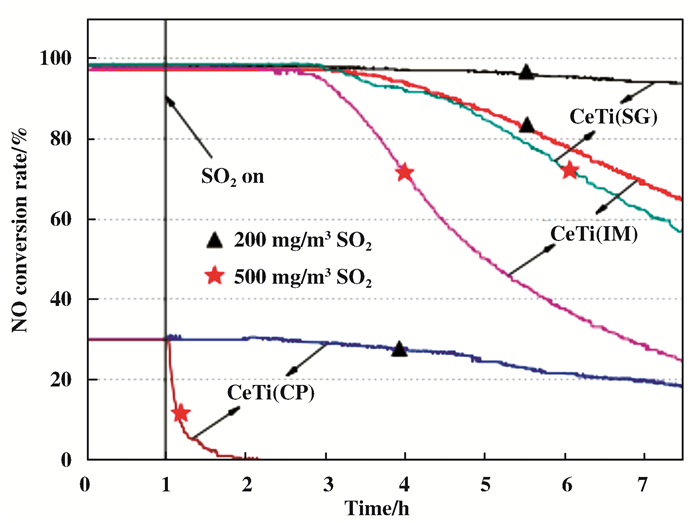
|
图 4 SO2存在下制备的催化剂活性 Fig.4 Catalyst activities prepared in the presence of SO2 |
载体物质与活性组分是SCR催化剂的两大组成部分[2, 32-33].其中, 载体物质目前最常见的是TiO2、SiO2和Al2O3等金属氧化物[2].活性组分主要有Pt、V、Ce、Pd和W[2].催化剂的催化活性, 是由载体与活性组分之间相互影响、相互作用而实现的[2, 34-38].对于活性组分的选择上, 由于Pt与Pd为贵金属, 在成本上远远高于V、Ce和W等, 且在高温下, 负载Pt与Pd也会导致NH3被非选择性氧化.因而, 在脱硝领域中通常会研究非贵金属催化剂[39-44].
针对催化剂的载体, Nam等[45]以P25TiO2为载体, 以浸渍法制备V2O5/TiO2、V2O5/Al2O32、V2O5/SiO和纯V2O5催化剂, 经活性测试, 上述催化剂均具有相对良好的催化活性, 其反应产物主要为N2及H2O.其中, V2O5/SiO2的催化活性相对较差.但当V2O5的负载量在5%(摩尔分数)以下时, 相对于V2O5/Al2O3, V2O5/TiO2具有较好的催化活性.进而得出TiO2更适于作为载体.与此同时, Lietti[46]和Svachula[47]等通过研究发现, V2O5不仅可以促进NOx还原, 还可以将SO2氧化成SO3. Amiridis等[48]认为V2O5负载量与催化剂的活性有着很好的线性关系, 负载量越高, 则催化剂的催化活性越强.但Saleh等[49]却发现高负载量的V2O5可能会致使锐钛矿相的TiO2向金红石相转变.这个过程中的相变则会降低催化剂的比表面积及催化活性.为避免TiO2的相变, 就必须控制V2O5的负载量. Djerad等[50]通过研究发现, 增加氧化钨的负载量可以避免TiO2的相变, 从而保持催化剂的高催化活性.同时, Choo[51]和李[12]等通过研究发现WO3还可以促使聚合钒酸盐在催化剂内形成, 而聚合的钒酸盐具有更加活泼的活性位点, 进而使催化剂具有更高的催化活性.
Amiridisa、Alemany和Kobayashi[52-54]等针对钨的含量对SCR催化性能的影响, 进行了深入研究和详细描述.他们发现钨含量的增加可以增强催化剂的催化效率.同时伴随反应温度的提升, 催化剂的催化活性也逐渐提高, 当温度达到450 ℃时, 其NO脱除效率可达到80%以上, 基于此, WO3的引入可以进一步拓宽催化剂的反应温宽.钨的含量为8%, 催化剂的催化活性最强, 随后, 催化剂的催化活性变成下降趋势[55].
研究发现, V的价态(V3+、V4+及V5+)对于SCR反应有着十分重要的作用和影响[2].钒基催化剂中的钒在载体表面上是单层分散的[56-57], 其表面不仅含有高价的钒(V5+), 同时也存在低价的钒(V3+和V4+).广泛运用DRIFT方法进行研究的结果表明, V2O5-WO3/TiO2催化剂的SCR反应机理主要属于E-R机理[58-61]. Inomata等[61]发现钒系催化剂的反应机理为E-R机理, 反应中需两个相临的V=O位点协同发挥其催化作用, NH3则吸附在上述两个位点上进而转化为NH4+, 随后与NO发生反应, 生成N2及H2O. Yin等[62]通过DFT理论计算进一步验证了上述结论.同时, E-R机理也涉及到了V4+与V5+的相互转换, 其包含NH3促使V5+=O形成+ H3-N……H-O-V4+的还原过程及H-O-V4+至V5+=O的氧化过程[63].
Dong等[64]发现V4+/V5+的数值对催化剂的催化活性有着重要的影响.其归结于V4+/V5+的数值可以影响酸性位点的数量, 进而影响催化剂的表观活化能, 同时也确保V4+(V3+)和V5 +之间的相互转换(表 1).同时, 煅烧过程中氧的含量也会显著影响V4+/V5+的数值, 从而进一步影响催化剂的催化活性.据此, 低价钒有助于改善催化剂中电子之间的相互传递[65-69].
| 表 1 催化剂表面原子价态及其占比 Table 1 Atomic valence state of catalyst surface and its proportion |
对于钒基催化剂来说, 其制备过程中工艺的改变, 也会大大影响催化剂的微观结构. Zhao等[70]曾对催化剂的制备顺序进行了研究, 经过研究后, 发现先负载钒后负载钨所制备出的催化剂其催化活性最好, 将其原因归结为先负载的成份会与载体的作用更强, 然而其研究的缺陷在于未保持负载活性组分比例的一致性, 故而该结论有所争议, 但是其理论的提出, 对于后续制备工序对脱硝催化剂催化性能的研究有着重要的指导性意义.
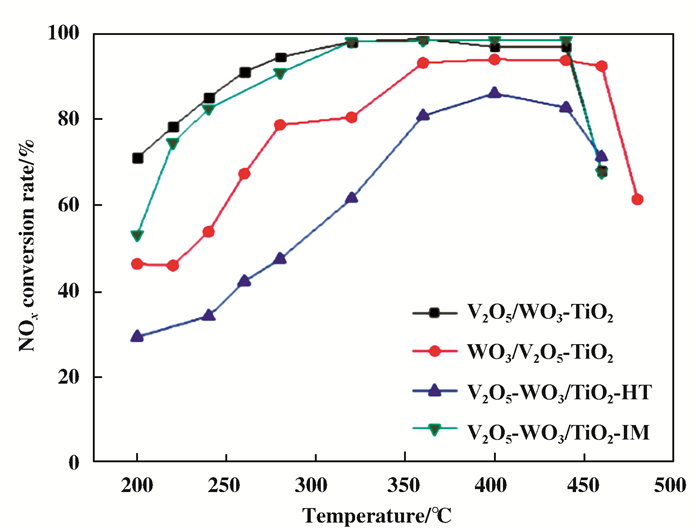
Zong等[71-73]针对催化剂的负载顺序进行了详细的研究, 主要包括:先负载钒, 后负载钨(WO3/V2O5-TiO2); 先负载钨, 后负载钒(V2O5/WO3-TiO2); 同时负载钒与钨V2O5-WO3/TiO2-HT; 以钛白粉为钛源, 同时负载钒与钨(V2O5-WO3/TiO2-IM) 4种催化剂, 并通过活性测试及表征分析对上述催化剂进行了详细研究.通过对催化剂活性的测试(图 5), 发现先负载钨, 后负载钒所制备的催化剂(V2O5/WO3-TiO2)具有最佳的反应活性.同时测试了该催化剂在SO2体积分数为300×10-6下的催化活性(图 6), 发现, 在200~240 ℃下, 催化剂的催化性能下降了23%, 随着温度的上升, 其催化活性的下降趋势逐渐减缓.
|
图 5 负载顺序对催化剂催化活性的影响 Fig.5 The effect of loading sequence on the catalytic activity of the catalyst |

|
图 6 V2O5/WO3-TiO2催化剂的抗硫性测试 Fig.6 V2O5/WO3-TiO2 catalyst sulfur resistance test a: no SO2; b: with SO2 |
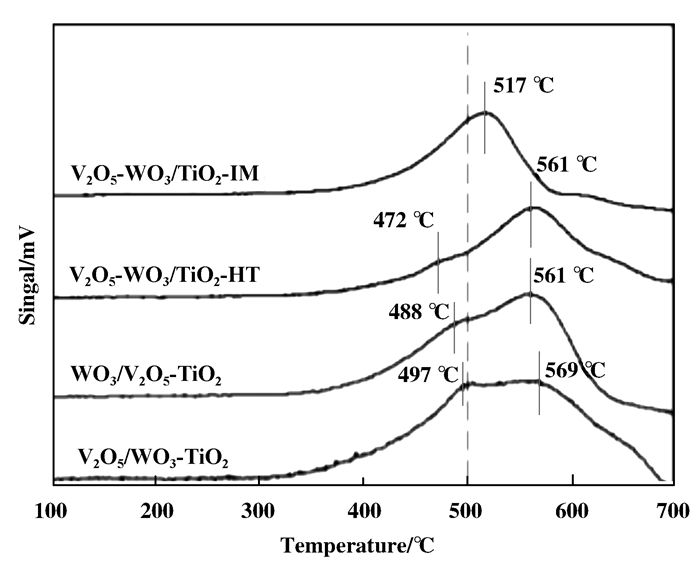
在H2-TPR分析结果(图 7)中, 发现在100~700 ℃的检测范围中, 催化剂均存在了两种还原峰.在400~500 ℃的还原峰是V5+向V4+的还原, 而500~570 ℃的还原峰则为V5+向V3+的还原.
|
图 7 不同催化剂的H2-TPR分析结果 Fig.7 H2-TPR analysis results of different catalysts |
该团队对通过XPS研究了钒的不同价态(表 2).其发现V4+/(V5++ V4++ V3+)表面原子比与催化活性有着明显的规律, 即先负载钨后负载钒制备的催化剂具有最高的V4+/(V5++ V4++ V3+)表面原子比, 结合E-R机制, 这也进一步佐证了V4+的存在可以进一步促进催化剂催化活性的提高.
| 表 2 不同催化剂的表面原子占比 Table 2 Proportion of surface atoms of different catalysts |
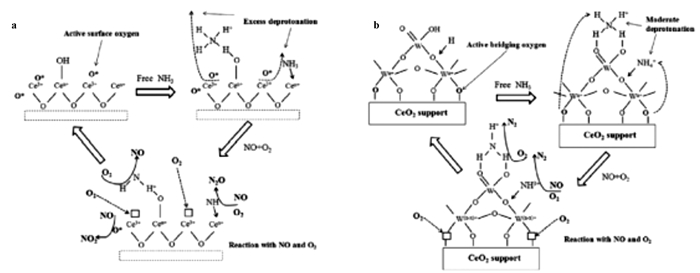
一系列研究表明, 铈基催化剂自身存在着良好的储氧能力, 其在SCR反应中是十分重要的[74-77].一般来说, Ce4+向Ce3+转变的过程可以释放氧气, 进而形成氧空位(如图 8), 而氧空位极易吸收氧分子.与此同时, Ce3+也会向Ce4+转变, 进而完成一个完整的催化循环[78-80]. Xu等[81]详细研究了CeO2负载量对Ce/TiO2催化剂活性的影响, 同时对SO2和H2O有着良好的耐受性, 即300 ℃, SO2和H2O的存在下, 经过12 h连续测试后催化剂催化活性仍可达到80%.需要说明的是, 铈基催化剂的催化效率也会受二氧化硫的影响.当其存在下, Ce与SO2会反应生成Ce2(SO4)3, 进而打破Ce3+与Ce4+之间的相互转变[82].相关研究表明, 在催化剂中掺杂一些其他过渡金属可以有效改善催化剂的抗水抗硫性[83-86].
|
图 8 催化剂的反应机理图 Fig.8 Reaction mechanism diagram of catalyst |
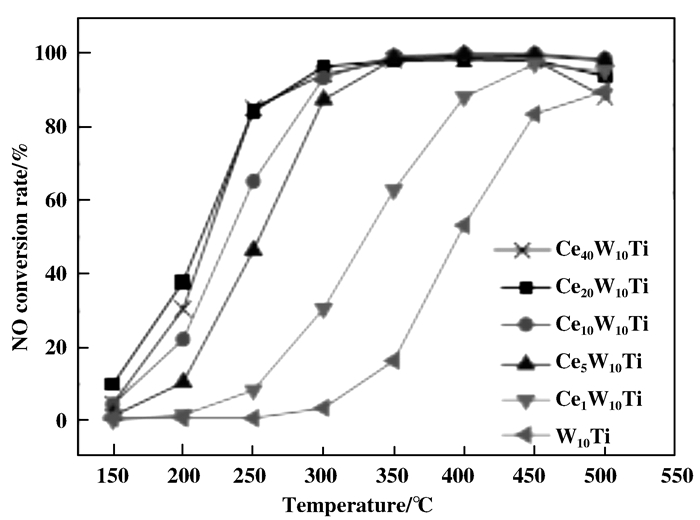
Jiang等[79]通过简单的溶胶凝胶法制备出了铈钛、钨钛和铈钨钛催化剂.首先通过活性测试分析Ce的负载量对催化剂催化活性的影响(如图 9), 从钨钛催化剂与铈钨钛催化剂的催化活性对比来看, 铈钨钛催化剂拥有更好的催化活性, 随着铈含量的增加, 铈钨钛催化剂的催化活性也逐渐增加, 但当铈的负载量超过20%时, 催化剂的催化活性有着下降的趋势, 故铈的负载量在20%时, 铈钨钛催化剂可以表现出最佳活性.
|
图 9 不同铈含量下催化剂的催化活性 Fig.9 Catalytic activities of catalysts with different cerium content |
随后该团队又对钨的负载量对催化剂催化活性的影响做了进一步的研究(如图 10), 其发现, 在300~450 ℃区间内, NO转化率基本保持在接近100%的范围内, 且随着钨负载量的变化, 其活性没有非常明显的改变.而在300 ℃以下, 450 ℃以上的温度范围内, NO转化率随着钨负载量的增加而增加, 尤其是当钨的负载量超过10%时, NO转化率有少量的改变.为进一步研究钨对铈钛催化剂SCR反应效率的影响, 该团队对催化剂进行了不同空速的活性测试及研究.随着空速的提升, 在300 ℃以下, 催化剂的催化活性有着很明显的下降; 当温度达到300 ℃时, 钨负载量为10%的铈钨钛催化剂具有最佳的SCR反应活性; 而在更高的温度条件下, 空速对催化剂催化活性的影响没有降低.

|
图 10 钨含量及空速对催化剂催化效率的影响 Fig.10 Effects of tungsten content and space velocity on catalyst efficiency |
同时该团队发现, 铈负载量为20%, 钨负载量为10%所制备出的催化剂具有最佳的抗水抗硫性(图 11).通过上述研究, 钨的负载可以进一步提高铈钛催化剂的催化活性及催化剂的抗水抗硫性.通过BET与XPS表征证实, 尽管铈钨钛催化剂的比表面积比铈钛催化剂的小, 但是钨的负载可以进一步促进铈在TiO2载体表面的分散, 增加Ce3+含量和化学氧含量, 而通过XRD与H2-TPR表征可以进一步证实钨、铈与二氧化钛之间的强相互作用可以极好的提高催化剂的催化活性.

|
图 11 铈含量为20%, 钨含量为10%所制备的催化剂的抗水抗硫性测试 Fig.11 Water and sulfur resistance tests of the prepared catalyst with cerium content of 20% and tungsten content of 10% |
一系列研究表明[87-97], 催化剂的催化活性及其稳定性也可以通过调节载体表面的性质来提高, 如形貌和比表面积. Zhou等[98]发现, 通过低温酸-水热过程可以合成不同纳米结构的二氧化钛:如球、花、棒和带等. Zhao等[99]通过合成纳米片TiO2(TiO2-NS)及纳米粒子TiO2(TiO2-NP), 发现以TiO2-NS为载体所制备的催化剂比以TiO2-NP为载体所制备的催化剂具有更佳的催化活性、稳定性及抗水抗硫性. Han等[100]合成了三维花状NiMnFe混合氧化物催化剂并作为NH3-SCR催化剂, 其团队发现三维花状结构的NiMnFe混合氧化物催化剂可以显著提高NH3-SCR的反应性能. Ma等[101]首次使用MOF作为前驱体以水热法合成了一系列的MOS2@TiO2纳米杂化物, 并将其用于催化氢的释放. Khalidet等[102]通过水热法合成了TiO2纳米粉, 发现随着水热时间的增加, 粉状结构的粒径增大. Wang等[103]以水热法合成了一系列不同形貌的二氧化钛载体(图 12).Tian等[104]也以TBOT为钛源, EG为助剂, 利用水热法制备出了花状TiO2.该团队发现, 水热时间的改变可以进一步改变TiO2的形状.同时, Chen等[105]也发现, TiO2的形态同样会被钛源的浓度所影响. Wang等[106]通过水热法合成了不同形状的TiO2, 发现花状二氧化钛的比表面积相对较大, 这也进一步提高了以其为载体所制备的催化剂的催化活性.
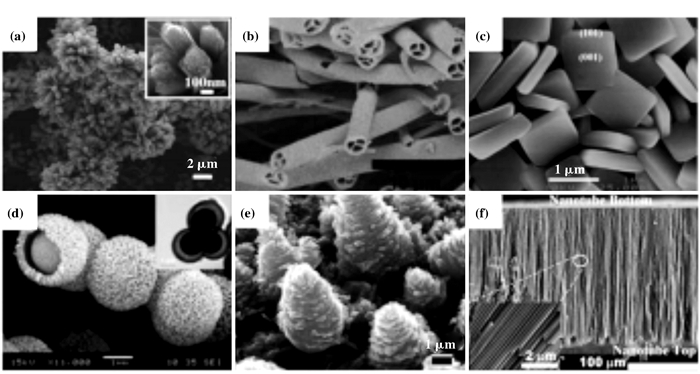
|
图 12 TiO2的SEM图 Fig.12 SEM images of TiO2 |
Zong等[107]采用水热法, 通过改变容积和水热温度制备出具有不同形貌的TiO2载体及催化剂.如图 13, 花状结构的TiO2载体是在160 ℃的水热条件下形成的.其根据水热温度与载体形状之间的关系, 研究了花状TiO2的形成机制.值得一提的是, 在120 ℃的水热温度下, 制备出了的棒状结的TiO2, 同时以该形状的TiO2为载体所制备的催化剂具有较高的催化活性.
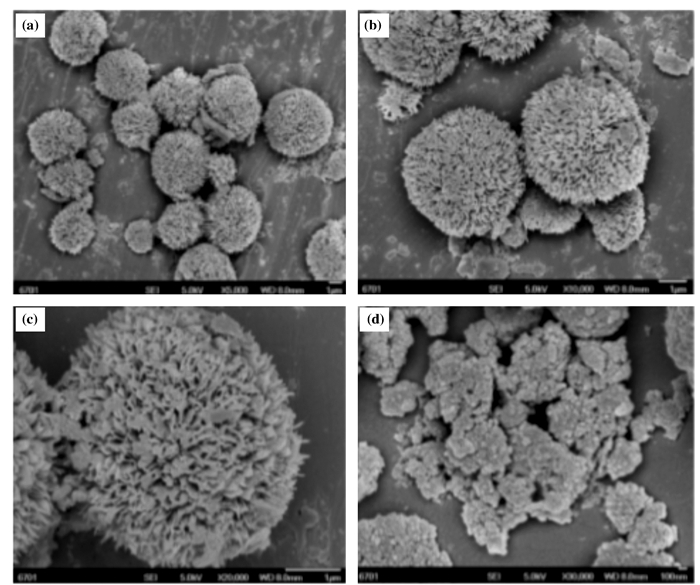
|
图 13 CeO2-WO3/TiO2-A-160催化剂的SEM图 Fig.13 SEM images of CeO2-WO3/TiO2-A-160 catalyst a: ×5000; b: ×10 000; c: ×20 000; d: CeO2-WO3/TiO2-W-160 |
为进一步研究其他条件对花状载体TiO2的影响, 在之前的研究基础上, Zong等[108]又通过改变不同的水热时间制备出了一系列载体TiO2, 并通过浸渍法合成CeO2-WO3/TiO2催化剂.当水热时间为8 h时, 所合成的催化剂具有最完整的三维花状结构(图 14), 且催化剂也表现出了最佳的催化性能及较好的抗水抗硫性(图 15).该催化剂的催化性能归结于催化剂中所含的较高的C3+/(C3++ C4+)表面原子比、活性组分的高度分散性及适当的酸性位点(图 16).
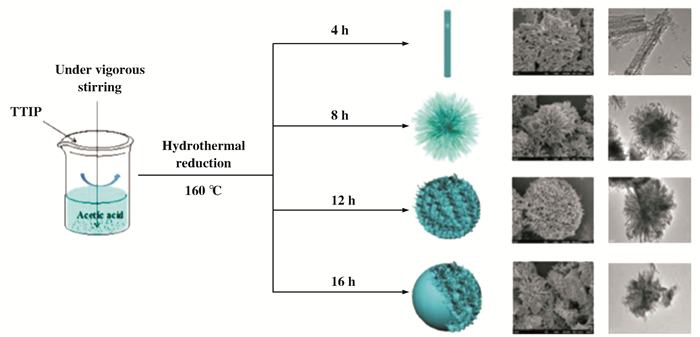
|
图 14 不同水热时间下制备的催化剂的SEM及TEM图 Fig.14 SEM and TEM images of the catalyst prepared under different hydrothermal time |
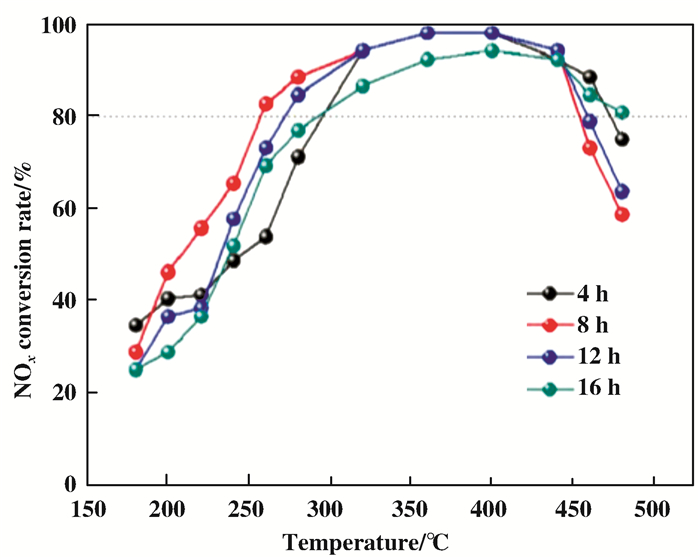
|
图 15 不同水热条件下制备的催化剂的催化活性 Fig.15 Catalytic activities of catalysts prepared under different hydrothermal conditions |

|
图 16 催化剂Ce3+/(Ce3++ Ce4+)含量与反应温度的关系图 Fig.16 Relationship between catalyst Ce3+ /(Ce3++ Ce4+) content and reaction temperature |
基于上述研究以及花状载体特殊的形貌及较大的比表面积等特点, 可以得出以水热法制备出花状载体, 进而用于制备脱硝催化剂将是一个很好的研究思路.
2.4 不同晶面TiO2载体对催化剂活性的影响值得一提的是, 对于锐钛矿TiO2载体材料, 其各晶面的平均表面能由低到高的顺序依次为: 0.44 J·m-2(101)<0.53 J·m-2(100)<0.90 J·m-2(001)[109].其不同晶面表面能的差异, 在世界范围内受到了关注[109-111]. (001)晶面表面的Ti—O—Ti角较大, 会导致键的失稳和表面氧原子的反应, 这些优点有利于催化活性[112-113].通过调控锐钛矿TiO2载体不同晶面的比例, 其反应活性也会发生相应的改变.因此, TiO2(001)晶面曝光率越高, 催化活性越好.由于这些优点, TiO2载体的改性也引起了许多研究者的关注[114-115].
Yang等[116]通过使用SEM、AFM、TEM、ED、XRD和XPS等表征方法在55~80 ℃下检查了熔融石英上锐钛矿二氧化钛的LPD基础.其团队发现, 在最初的非均相成核过程中, (110)轴垂直于玻璃基板[117].由于均匀成核速率的增加, 其细长的晶体通过生长融合在一起, 随后转变成圆形的花状晶岛.另外, 不同pH和温度下的实验也被进一步研究.在低pH条件下, 晶体的成核和生长均受阻, 而在较高的pH条件下, 晶体的成核和生长受到了极大的促进.
Deng等[118]合成具有(001)晶面的MnOx/TiO2 (NS), 该方法对NO的SCR反应比传统的(101)晶面的MnOx/TiO2 (NP)具有更好的催化活性(图 17). Huang等[119]还发现, (001)晶面的TiO2纳米薄片(TiO2-ns)的SCR性能比(101)平面的TiO2纳米颗粒(TiO2-np)要好得多.通过DRIFTS和DFT计算发现(001)晶面具有更高的表面能和更多的活性氧种类, 有利于NO的吸附和NO2的形成. Wang等[120]采用水热法合成了表面暴露(001)的二氧化钛, 并将其作为NH3-SCR氧化铈的催化剂载体.他们发现(001)高能面可以提高二氧化铈的热稳定性.基于以上研究, 水热法通常用于研究TiO2 (001)晶面的形成, 但是否有其他方法可以用于TiO2 (001)晶面的研究是一个有趣的方向.
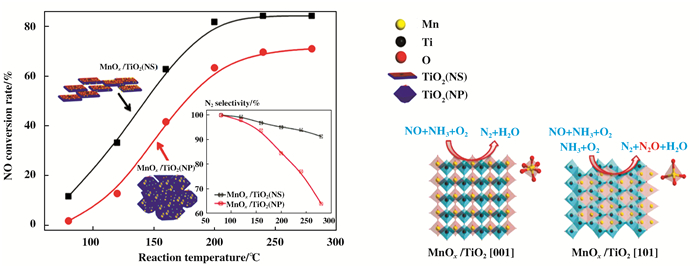
|
图 17 纳米催化剂的催化活性图 Fig.17 Nano-catalyst activity map |
在TiO2的制备中, 蒸发诱导自组装法(EISA)以低成本在经济上占据着很大的优势.此外, EISA法合成的催化剂还具有介孔膜和高表面积等优点[121-124]. Arconada等[125]通过EISA法合成了介孔TiO2并发现TiO2暴露了较高的表面积. Sarkar等[126]通过控制合成变量(膨胀剂/F127)浓度制备了TiO2, 发现孔径变化对不同的应用具有重要意义. Zong等[127]采用EISA法, 通过调节P123与TiO2的比例, 一步合成了的具有TiO2(001)晶面CeO2-WO3/TiO2催化剂, 并用于SCR反应.如图 18及图 19所示, 发现TiO2(001)晶面占比取决于P123与TiO2的比例, 且进一步决定了催化剂的催化活性.当P123与TiO2的比例为0.02时, 催化剂表现出最佳的SCR活性和极好的抗水抗硫性.这归咎于该比例下高占比的TiO2(001)晶面.
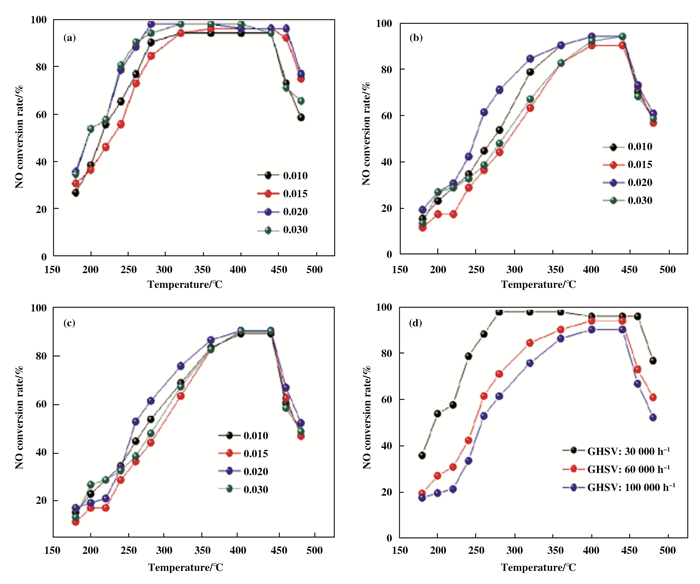
|
图 18 a:不同P123与TiO2的比例所制备的催化剂; b: 60 000 h-1空速下催化剂的催化活性; c: 100 000 h-1空速下催化剂的催化活性; d: P123与TiO2的比例为0.02下制备的催化剂在不同空速下的催化活性 Fig.18 a: catalyst prepared with different ratios of P123 and TiO2; b: catalytic activity of the catalyst at 60 000 h-1 space velocity; c: catalytic activity of the catalyst at 100 000 h-1 space velocity; d: catalytic activity of the catalyst at different space velocities which prepared at a ratio of P123 to TiO2 of 0.02 |
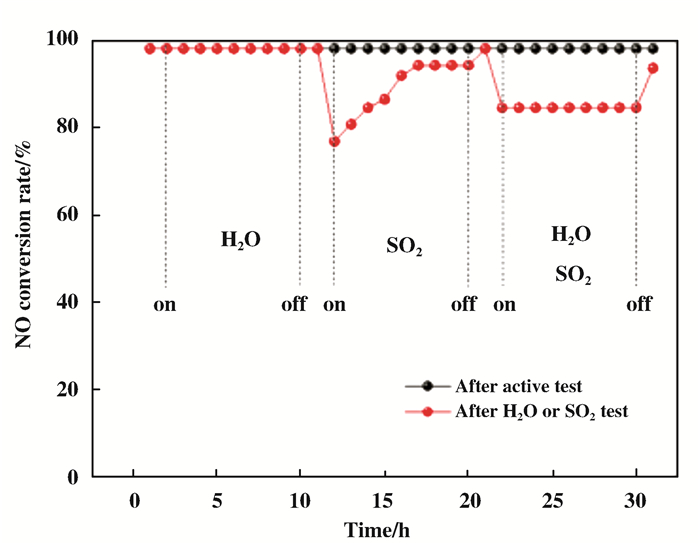
|
图 19 P123与TiO2的比例为0.02下制备的催化剂的抗水抗硫性测试结果 Fig.19 Results of water and sulfur resistance of the catalyst prepared under the ratio of P123 to TiO2 of 0.02 |
综上所述, 采用EISA方法研究TiO2的(001)面, 这可能更有利于提高催化活性.
3 结论目前, 对于脱硝催化剂领域, 应用最多的活性组分为钒、铈、锰等, 而对于其载体来说, 二氧化钛无疑是最佳选择.无论是载体还是活性组分的研究, 都将有助于催化剂催化性能的提高.通过不同的制备方法所得到的催化剂在催化性能、抗水抗硫性及其催化剂自身结构、表面形貌以及晶面等都存在着巨大的差异.制备方法的组合是目前最为流行的方向.不同载体结构对催化剂催化效率的影响中, 也需要考虑并解决以下几个实际工程应用中所存在的问题:
(1) 可替代型的TiO2载体; (2)克服目前工程应用中催化剂的催化温度高、温宽窄及催化活性低的问题; (3)增强催化剂在高水、高硫条件下的催化性能.脱硝催化剂研究的主要任务是筛选出最优的载体物质及活性组分, 采用最适合的制备方法, 制备出高催化活性、高温宽型及强抗水抗硫性的高效脱硝催化剂, 使其能够在工业上得到良好广泛的应用, 节约成本.
| [1] |
J Dumesic J A, Topsoe N Y, Topsoe H, et al. Kinetics of selective catalytic reduction of nitric oxide by ammonia over vanadia/titania[J]. J Catal, 1996, 163(2): 409–417.
DOI:10.1006/jcat.1996.0342 |
| [2] |
Zong Lu-yao (宗路遥), Zhang Ji-yi (张继义), Tang Zhi-cheng (唐志诚). Influence mechanism of support structure on catalytic performance for NH3-SCR reaction (脱硝催化剂载体结构对催化性能的影响机制)[D]. Lanzhou (兰州): Lanzhou University of Technology (兰州理工大学), 2018.
|
| [3] |
Xiao Jun(肖俊), Yang Hai-yu (杨海余). Research on the strategy and plan of NS corporation (NS公司的发展战略研究)[D]. Changsha (长沙): Changsha University of Science & Technology (长沙理工大学), 2017.
http://cdmd.cnki.com.cn/Article/CDMD-10141-1015560635.htm |
| [4] |
Klukowski D, Balle P, Geiger B, et al. On the mechanism of the SCR reaction on Fe/HBEA zeolite[J]. Appl Catal B:Environ, 2009, 93(1/2): 185–193.
|
| [5] |
Kwak J H, Tran D, Burton S D, et al. Effects of hydrothermal aging on NH3-SCR reaction over Cu/zeolites[J]. J Catal, 2012, 287: 203–209.
DOI:10.1016/j.jcat.2011.12.025 |
| [6] |
Busca G, Lietti L, Ramis G, et al. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts:A review[J]. Appl Catal B:Environ, 1998, 18(1/2): 1–36.
|
| [7] |
Huang Z, Liu Z, Zhang X, et al. Inhibition effect of H2O on V2O5/AC catalyst for catalytic reduction of NO with NH3 at low temperature[J]. Appl Catal B:Environ, 2006, 63(3/4): 260–265.
|
| [8] |
Wang X, Shi A, Duan Y, et al. Catalytic performance and hydrothermal durability of CeO2-V2O5-ZrO2/WO3-TiO2 based NH3-SCR catalysts[J]. Catal Sci Technol, 2012, 2(7): 1386–1395.
DOI:10.1039/c2cy20140b |
| [9] |
Qiu Y, Liu B, Du J, et al. The monolithic cordierite supported V2O5-MoO3/TiO2 catalyst for NH3-SCR[J]. Chem Eng J, 2016, 294: 264–272.
DOI:10.1016/j.cej.2016.02.094 |
| [10] |
Shimizu K, Satsuma A. Selective catalytic reduction of NO over supported silver catalysts-practical and mechanistic aspects[J]. Phys Chem Chem Phys, 2006, 89(23): 2677–2695.
|
| [11] |
Xu Chao-chao (徐超超), Yang Ji (杨骥). Highly efficient spinel catalysts for the selective catalytic reduction of NO with H2 at low temperature (高效低温尖晶石型催化剂用于氢气选择性催化还原NO的研究)[D]. Shanghai (上海): East China University of Science and Technology (华东理工大学), 2016.
http://cdmd.cnki.com.cn/Article/CDMD-10251-1016097416.htm |
| [12] |
Li Pan-pan(李盼盼), Yu Feng(于锋), Zhu Ming-yuan(朱明远), et al. Selective catalytic reduction De-NOx catalysts(选择性氧化还原脱硝催化剂)[J]. Process Chem(化学进展), 2016, 28(10): 1578–1590.
|
| [13] |
Yin Ye-shan (尹业彬), Gou Xiang (苟湘). Experimental study and numerical simulation of fluidized bed SCR reactor (流化床SCR反应器的实验研究及数值模拟)[D]. Tianjin (天津): Hebei University of Technology (河北工业大学), 2015.
http://cdmd.cnki.com.cn/Article/CDMD-10080-1017873554.htm |
| [14] |
Xie Li-juan(谢丽娟), Shi Xiao-yan(石晓燕), Liu Fu-dong(刘福东), et al. Selective catalytic reduction of NOx from diesel engine with NH3 over zeolites catalysts with chabazite(菱沸石在柴油车尾气NOx催化净化中的应用)[J]. Process Chem(化学进展), 2016, 28(12): 1860–1869.
DOI:10.7536/PC160731 |
| [15] |
Xu Li-li (徐丽丽), Shen Mei-qing (沈美庆), Jia Li-wei (贾莉伟). The effect of synthesis methods on Ce species and NH3-SCR activity over V/Ce/WTi (合成方法对V/Ce/WTi催化剂Ce物种及NH3-SCR性能的影响研究)[D]. Tianjin (天津): Tianjin University (天津大学), 2016.
http://cdmd.cnki.com.cn/Article/CDMD-10056-1017134050.htm |
| [16] |
Han L, Cai S, Gao M, et al. Selective catalytic reduction of NOx with NH3 by using novel catalysts:State of the art and future prospects[J]. Chem Rev, 2019, 119: 10916–10976.
DOI:10.1021/acs.chemrev.9b00202 |
| [17] |
Gillot S, Tricot G, Vezin H, et al. Development of stable and efficient CeVO4 systems for the selective reduction of NOx by ammonia:Structure-activity relationship[J]. Appl Catal B:Environ, 2017, 218: 338–348.
DOI:10.1016/j.apcatb.2017.06.049 |
| [18] |
Liu B, Liu J, Ma S, et al. Mechanistic study of selective catalytic reduction of NO with NH3 on W-doped CeO2 catalysts:Unraveling the catalytic cycle and the role of oxygen vacancy[J]. J Phys Chem C, 2016, 120(4): 2271–2283.
DOI:10.1021/acs.jpcc.5b11355 |
| [19] |
Li X, Li Y. Molybdenum modified CeAlOx catalyst for the selective catalytic reduction of NO with NH3[J]. J Mol Catal A:Chem, 2014, 386: 69–77.
DOI:10.1016/j.molcata.2014.02.016 |
| [20] |
Lan Yi (兰益), Liu Xiao-dong (刘晓东), Yang Shi-jian (杨士建). Collaborative denitration and removal of gaseous elemental mercury on Mn-Fe spinel (Mn-Fe尖晶石协同脱硝除汞研究)[D]. Nanjing (南京): Nanjing University of Science & Technology (南京理工大学), 2015.
http://cdmd.cnki.com.cn/Article/CDMD-10288-1015365645.htm |
| [21] |
Yang S, Fu Y, Liao Y, et al. Competition of selective catalytic reduction and non selective catalytic reduction over MnOx/TiO2 for NO removal:The relationship between gaseous NO concentration and N2O selectivity[J]. Catal Sci & Technol, 2014, 4: 224–232.
|
| [22] |
Tang X, Li J, Sun L, et al. Origination of N2O from NO reduction by NH3 over β-MnO2 and α-Mn2O3[J]. Appl Catal B:Environ, 2010, 99(1): 156–162.
|
| [23] |
Liu F, He H J, Zhang C. Mechanism of the selective ca-talytic reduction of NOx with NH3 over environmental-friendly iron titanate catalyst[J]. Catal Today, 2011, 175(1): 18–25.
DOI:10.1016/j.cattod.2011.02.049 |
| [24] |
Ding S, Liu F, Shi X. Significant promotion effect of Mo additive on novel Ce-Zr mixed oxide catalyst for the selective catalytic reduction of NOx with NH3[J]. ACS Appl Mater & Inter, 2015, 7(18): 9497–9506.
|
| [25] |
Liu F, He H, Zhang C, et al. Selective catalytic reduction of NO with NH3 over iron titanate catalyst:Catalytic performance and characterization[J]. Appl Catal B:Environ, 2010, 96(3/4): 408–420.
|
| [26] |
Liu F, He H. Structure-activity relationship of iron tita-nate catalysts in the selective catalytic reduction of NOx with NH3[J]. J Phys Chem C, 2010, 114(40): 16929–16936.
DOI:10.1021/jp912163k |
| [27] |
Qi G, Yang R, Chang R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures[J]. Appl Catal B:Environ, 2004, 51(2): 93–106.
DOI:10.1016/j.apcatb.2004.01.023 |
| [28] |
Wu Shi-guo (伍士国), Dong Lin (董林). The research of performance of FeMnTiOx mixed oxides catalyst by CTAB-assisted in the NH3-selective catalytic reduction for the denitration of stationary sources(基于CTAB辅助制备的FeMnTiOx催化剂NH3-SCR脱硝的性能研究)[D]. Nanjing (南京): Nanjing University (南京大学), 2015.
http://d.wanfangdata.com.cn/Thesis/Y2949607 |
| [29] |
Gao X, Jiang Y, Fu Y, et al. Preparation and characte-rization of CeO2/TiO2 catalysts for selective catalytic reduction of NO with NH3[J]. Catal Commun, 2010, 11(5): 465–469.
DOI:10.1016/j.catcom.2009.11.024 |
| [30] |
Li Lu-lu (李露露), Dong Lin (董林). The research of preparation and performance of ceria-based catalyst in the NH3-selective catalytic reduction (铈基NH3-SCR催化剂的制备及其脱硝性能研究)[D]. Nanjing (南京): Nanjing University (南京大学), 2017.
http://www.cqvip.com/QK/90630X/201301/45004086.html |
| [31] |
Ma Lin (马琳), Chang Li-ping (常丽萍), Liao Jun-jie (廖俊杰). Study on AlCl3 loaded catalyst of alkylation reaction thiophene and olefin in coking benzene (焦化苯中噻吩与烯烃烷基化反应用AlCl3负载型催化剂的研究)[D]. Taiyuan(太原): Taiyuan University of Technology (太原理工大学), 2014.
http://d.wanfangdata.com.cn/Thesis/Y2693643 |
| [32] |
Lietti L, Forzatti P, Bregani F. Steady-state and transient reactivity study of TiO2-supported V2O5-WO3 De-NOx catalysts:Relevance of the vanadium-tungsten interaction on the catalytic activity[J]. Indus Eng Chem Res, 1996, 35(11): 3884–3892.
DOI:10.1021/ie960158l |
| [33] |
Peng Y, Li J, Si W, et al. In sight into deactivation of commercial SCR catalyst by arsenic:An experiment and DFT study[J]. Environ Sci Technol, 2014, 48(23): 13895–13900.
DOI:10.1021/es503486w |
| [34] |
Kleemann M, Elsener M, Koebel M, et al. Hydrolysis of isocyanic acid on SCR catalysts[J]. Indust Eng Chem Res, 2000, 39(11): 4120–4126.
DOI:10.1021/ie9906161 |
| [35] |
Yan N, Chen W, Chen J, et al. Significance of RuO2 modified SCR catalyst for elemental mercury oxidation in coal-fired flue gas[J]. Environ Sci Technol, 2011, 45(13): 5725–5730.
DOI:10.1021/es200223x |
| [36] |
Kamata H, Ueno S, Naito T, et al. Mercury oxidation over the V2O5(WO3)/TiO2 commercial SCR catalyst[J]. Indus Eng Chem Res, 2008, 47(21): 8136–8141.
DOI:10.1021/ie800363g |
| [37] |
Peng Y, Li J, Shi W, et al. Design strategies for development of SCR catalyst:Improvement of alkali poisoning resistance and novel regeneration method[J]. Environ Sci Technol, 2012, 46(22): 12623–12629.
DOI:10.1021/es302857a |
| [38] |
Negreira A S, Wilcox J. Role of WO3 in the Hg oxidation across the V2O5-WO3-TiO2 SCR catalyst:A DFT study[J]. J Phys Chem C, 2013, 117(46): 24397–24406.
DOI:10.1021/jp407794g |
| [39] |
Zhao W, Zhong Q, Zhang T, et al. Characterization study on the promoting effect of F-doping V2O5/TiO2 SCR catalysts[J]. RSC Adv, 2012, 2(20): 7906–7914.
DOI:10.1039/c2ra20987j |
| [40] |
Liu F, Yu Y, He H. Environmentally-benign catalysts for the selective catalytic reduction of NOx from diesel engines:Structure-activity relationship and reaction mechanism aspects[J]. Chem Commun, 2014, 45(36): 8445–8463.
|
| [41] |
Yang S, Wang C, Ma L, et al. Substitution of WO3 in V2O5/WO3-TiO2 by Fe2O3 for selective catalytic reduction of NO with NH3[J]. Catal Sci Technol, 2013, 3(1): 161–168.
DOI:10.1039/C2CY20383A |
| [42] |
Qu R, Ye D, Zheng C, et al. Exploring the role of V2O5 in the reactivity of NH4HSO4 with NO on V2O5/TiO2 SCR catalysts[J]. RSC Adv, 2016, 6(104): 102436–102443.
DOI:10.1039/C6RA22571C |
| [43] |
Li P, Xin Y, Li Q, et al. Ce-Ti Amorphous oxides for selective catalytic reduction of NO with NH3:Confirmation of Ce-O-Ti active sites[J]. Environ Sci Tech, 2012, 46(17): 9600–9605.
DOI:10.1021/es301661r |
| [44] |
Li X, Li J, Peng Y, et al. Comparison of the structures and mechanism of arsenic deactivation of CeO2-MoO3 and CeO2-WO3 SCR catalysts[J]. J Phys Chem C, 2016, 120(32): 18005–18014.
DOI:10.1021/acs.jpcc.6b03687 |
| [45] |
Nam I, Eldrldge J W, Klttrell J R. Deactivation of a vanadia-alumina catalyst for nitric oxide reduction by NH3[J]. Indus Eng Chem Prod Res Development, 1986, 25(2): 192–197.
DOI:10.1021/i300022a012 |
| [46] |
Lietti L, Nova I, Forzatti P. Selective catalytic reduction (SCR) of NO by NH3 over TiO2-supported V2O5-WO3 and V2O5-MoO3 catalysts[J]. Topics Catal, 2000, 12(1/4): 111–122.
|
| [47] |
Svachula J, Alemany L, Ferlazzo N, et al. Oxidation of sulfur dioxide to sulfur trioxide over honeycomb DeNoxing catalysts[J]. Indus Eng Chem Res, 1993, 32(5): 826–834.
DOI:10.1021/ie00017a009 |
| [48] |
Amiridis M D, Solar J P. Selective catalytic reduction of nitric oxide by ammonia over V2O5/TiO2, V2O5/TiO2/SiO2, and V2O5-WO3/TiO2 catalysts:Effect of vanadia content on the activation energy[J]. Indus Eng Chem Res, 1996, 35(3): 978–981.
DOI:10.1021/ie950452y |
| [49] |
Saleh R, Wachs I, Chan S, et al. The interaction of V2O5 with TiO2 (anatase):Catalyst evolution with calcination temperature and O-xylene oxidation[J]. J Catal, 1986, 98(1): 102–114.
|
| [50] |
Djerad S, Tifouti L, Crocoll M, et al. Effect of vanadia and tungsten loadings on the physical and chemical cha-racteristics of V2O5-WO3/TiO2 catalysts[J]. J Mol Catal A:Chem, 2004, 208(1/2): 257–265.
|
| [51] |
Choo S, Yim D, Nam I, et al. Effect of promoters including WO3 and BaO on the activity and durability of V2O5/sulfated TiO2 catalyst for NO reduction by NH3[J]. Appl Catal B:Environ, 2003, 44(3): 237–252.
DOI:10.1016/S0926-3373(03)00073-0 |
| [52] |
Amiridisa M D, Duevelb R V, Wachs I E. The effect of metal oxide additives on the activity of V2O5/TiO2 catalysts for the selective catalytic reduction of nitric oxide by ammonia[J]. Appl Catal B:Environ, 1999, 20(2): 111–122.
DOI:10.1016/S0926-3373(98)00101-5 |
| [53] |
Alemany L J, Lietti L, Ferlazzo N, et al. Reactivity and physicochemical characterization of V2O5-WO3/TiO2 De-NOx catalysts[J]. J Catal, 1995, 155(1): 117–130.
|
| [54] |
Kobayashi M, Hagi M. V2O5-WO3/TiO2-SiO2-SO42- catalysts:Influence of active components and support on activities in the selective catalytic reduction of NO by NH3 and in the oxidation of SO2[J]. Appl Catal B:Environ, 2006, 63(1/2): 104–113.
|
| [55] |
Hu Jian-fei (胡建飞), Song Kai (宋凯). Research on denitrification performance of titanium-based SCR catalysts (钛基SCR催化剂及其脱硝性能研究)[D]. Taiyuan (太原): Taiyuan University of Technology (太原理工大学), 2011.
|
| [56] |
Burkardt A, Weiweiler W, Tillaart J, et al. Influence of the V2O5 loading on the structure and activity of V2O5/TiO2 catalysts for vehicle application[J]. Topics Catal, 2016, 16(1/4): 369–375.
|
| [57] |
Tang F, Zhuang K, Yang L, et al. Effect of dispersion state and surface properties of supported vanadia on the activity of V2O5/TiO2 catalysts for the selective catalytic reduction of NO by NH3[J]. Chin J Catal, 2012, 33(4/6): 933–940.
|
| [58] |
Xiong S, Liao Y, Dang H, et al. Promotion mechanism of CeO2 addition on the low temperature SCR reaction over MnOx/TiO2:A new insight from the kinetic study[J]. RSC Adv, 2015, 5(35): 27785–27793.
DOI:10.1039/C5RA01767J |
| [59] |
Xiong S, Liao Y, Xiao X, et al. Novel effect of H2O on the low temperature selective catalytic reduction of NO with NH3 over MnOx-CeO2:Mechanism and kinetic study[J]. J Phys Chem C, 2015, 119(8): 4180–4208.
DOI:10.1021/jp512407k |
| [60] |
Hu P, Huang Z, Hua W, et al. Effect of H2O on cataly-tic performance of manganese oxides in NO reduction by NH3[J]. Appl Catal A:General, 2012, 437/438: 139–148.
DOI:10.1016/j.apcata.2012.06.025 |
| [61] |
Inomata M, Miyamoto A, Murakami Y. Mechanism of the reaction of NO and NH3 on vanadium oxide catalyst in the presence of oxygen under the dilute gas condition[J]. J Catalysis, 1980, 62(1): 140–148.
DOI:10.1016/0021-9517(80)90429-7 |
| [62] |
Yin X, Han H, Gunji I, et al. NH3 adsorption on the Bronsted and Lewis acid sites of V2O5(010):A periodic density functional study[J]. J Phys Chem B, 1999, 103(22): 4701–4706.
DOI:10.1021/jp990363p |
| [63] |
Zhi B, Ding H, Wang D, et al. Ordered mesoporous MnO2 as a synergetic adsorbent for effective arsenic(Ⅲ) removal[J]. J Mater Chem A, 2014, 2(7): 2374–2382.
DOI:10.1039/c3ta13790b |
| [64] |
Dong G, Bai Y, Zhang Y, et al. Effect of the V4+(3+)/V5+ ratio on the denitration activity for V2O5-WO3/TiO2 catalysts[J]. New J Chem, 2015, 39(5): 3588–3596.
DOI:10.1039/C5NJ00015G |
| [65] |
Gan L, Gao F, Yu J, et al. Improve low-temperature activity of V2O5-WO3/TiO2 for denitration using different vanadium precursors[J]. J Catal, 2016, 6(2): 25–39.
|
| [66] |
Dong G, Zhang Y, Zhao Y, et al. Effect of the pH value of precursor solution on catalytic performance of V2O5-WO3/TiO2 in the low temperature NH3-SCR of NOx[J]. J Fuel Chem Technol, 2014, 42(12): 1455–1463.
DOI:10.1016/S1872-5813(15)60003-2 |
| [67] |
Sa Y J, Kwon K, Cheon J Y, et al. Ordered mesoporous Co3O4 spinels as stable, bifunctional, noble metal-free oxygen electrocatalysts[J]. J Mater Chem A, 2013, 1(34): 9992–10001.
DOI:10.1039/c3ta11917c |
| [68] |
Zhan S, Zhu D, Qiu M, et al. Highly efficient removal of NO with ordered mesoporous manganese oxide at low temperature[J]. RSC Adv, 2015, 5(37): 29353–29361.
DOI:10.1039/C4RA17300G |
| [69] |
Kwon D W, Park K H, Hong S C. The influence on SCR activity of the atomic structure of V2O5/TiO2 catalysts prepared by a mechanochemical method[J]. Appl Catal A:General, 2013, 451: 227–235.
DOI:10.1016/j.apcata.2012.09.050 |
| [70] |
Zhao K, Han W, Tang Z, et al. Investigation of coating technology and catalytic performance over monolithic V2O5-WO3/TiO2 catalyst for selective catalytic reduction of NOx with NH3[J]. Coll Surf A:Physicochem Eng Aspects, 2016, 503: 53–60.
DOI:10.1016/j.colsurfa.2016.05.014 |
| [71] |
Zong L, Dong F, Zhang G, et al. Highly efficient mesoporous V2O5/WO3-TiO2 catalyst for selective catalytic reduction of NOx:Effect of the valence of V on the cataly-tic performance[J]. Catal Sur Asia, 2017, 21(3): 101–113.
|
| [72] |
Yang S, Wang C, Ma L, et al. Substitution of WO3 in V2O5/WO3-TiO2 by Fe2O3 for selective catalytic reduction of NO with NH3[J]. Catal Sci Technol, 2013, 3(1): 161–168.
DOI:10.1039/C2CY20383A |
| [73] |
Li X, Li J, Peng Y, et al. Selective catalytic reduction of NO with NH3 over novel iron-tungsten mixed oxide catalyst in a broad temperature range[J]. Catal Sci Technol, 2015, 5(9): 4556–4564.
DOI:10.1039/C5CY00605H |
| [74] |
Peng Y, Si W, Li X, et al. Investigation of the poisoning mechanism of lead on the CeO2-WO3 catalyst for the NH3-SCR reaction via in situ IR and Raman spectroscopy measurement[J]. Environ Sci Technol, 2016, 50(17): 9576–9582.
DOI:10.1021/acs.est.6b02307 |
| [75] |
Ding J, Zhong Q, Zhang S. A new insight into catalytic ozonation with nanosized Ce-Ti oxides for NOx removal:Confirmation of Ce-O-Ti for active sites[J]. Indus Eng Chem Res, 2015, 54(7): 2012–2022.
DOI:10.1021/ie504100b |
| [76] |
Geng Y, Shan W, Xiong S, et al. Effect of CeO2 for a high-efficiency CeO2/WO3-TiO2 catalyst on N2O formation in NH3-SCR:A kinetic study[J]. Catal Sci Technol, 2016, 6(9): 3149–3155.
DOI:10.1039/C5CY01968K |
| [77] |
Tang C, Zhang H, Dong L. Ceria-based catalysts for low-temperature selective catalytic reduction of NO with NH3[J]. Catal Sci Technol, 2016, 6(5): 1248–1264.
DOI:10.1039/C5CY01487E |
| [78] |
Gao X, Jiang Y, Fu Y, et al. Preparation and characte-rization of CeO2/TiO2 catalysts for selective catalytic reduction of NO with NH3[J]. Catal Commun, 2010, 11(5): 465–469.
DOI:10.1016/j.catcom.2009.11.024 |
| [79] |
Jiang Y, Xing Z, Wang X, et al. Activity and characte-rization of a Ce-W-Ti oxide catalyst prepared by a single step sol-gel method for selective catalytic reduction of NO with NH3[J]. Fuel, 2015, 151: 124–129.
DOI:10.1016/j.fuel.2015.01.061 |
| [80] |
Zhang Q, Liu X, Ning P, et al. Enhanced performance in NOx reduction by NH3 over a mesoporous Ce-Ti-MoOx catalyst stabilized by a carbon template[J]. Catal Sci Technol, 2015, 5(4): 2260–2269.
DOI:10.1039/C4CY01371A |
| [81] |
Xu W, Yu Y, Zhang C, et al. Selective catalytic reduction of NOx with NH3 over a Ce/TiO2 catalyst[J]. Catal Commun, 2008, 9(6): 1453–1457.
DOI:10.1016/j.catcom.2007.12.012 |
| [82] |
Jiang Y, Xing Z, Wang X, et al. Preparation and cha-racterization of CeO2/TiO2 catalysts for selective catalytic reduction of NO with NH3[J]. Catal Commun, 2010, 11(5): 465–469.
DOI:10.1016/j.catcom.2009.11.024 |
| [83] |
Xu W, He H, Yu Y. Deactivation of a Ce/TiO2 catalyst by SO2 in the selective catalytic reduction of NO by NH3[J]. J Phys Chem C, 2009, 113(11): 4426–4432.
DOI:10.1021/jp8088148 |
| [84] |
Michalow-Mauke K A, Lu Y, Kowalski K, et al. Flame-made WO3/CeOxTiO2 catalysts for selective catalytic reduction of NOx by NH3[J]. ACS Catal, 2015, 5(10): 5657–5672.
DOI:10.1021/acscatal.5b01580 |
| [85] |
Iwasaki M, Iglesia E. Mechanistic assessments of NO oxidation turnover rates and active site densities on WO3-promoted CeO2 catalysts[J]. J Catal, 2016, 342: 84–97.
DOI:10.1016/j.jcat.2016.07.011 |
| [86] |
Li X, Li X, Chen J, et al. An efficient novel regeneration method for Ca-poisoning V2O5-WO3/TiO2 catalyst[J]. Catal Commun, 2016, 87: 45–48.
DOI:10.1016/j.catcom.2016.06.017 |
| [87] |
Shan W, Liu F, He H, et al. A superior Ce-W-Ti mixed oxide catalyst for the selective catalytic reduction of NOx with NH3[J]. Appl Catal B:Environ, 2012, 115/116: 100–106.
DOI:10.1016/j.apcatb.2011.12.019 |
| [88] |
Shan W, Liu F, He H, et al. Novel cerium-tungsten mixed oxide catalyst for the selective catalytic reduction of NOx with NH3[J]. Chem Commun, 2011, 47(28): 8046–8048.
DOI:10.1039/c1cc12168e |
| [89] |
Shan W, Liu F, Yu Y, et al. The use of ceria for the selective catalytic reduction of NOx with NH3[J]. Chin J Catal, 2014, 35(8): 1251–1259.
DOI:10.1016/S1872-2067(14)60155-8 |
| [90] |
Huang H, Liu K, Chen K, et al. Ce and F comodification on the crystal structure and enhanced photocatalytic activity of Bi2WO6 photocatalyst under visible light irradiation[J]. J Phys Chem C, 2014, 118(26): 14379–14387.
DOI:10.1021/jp503025b |
| [91] |
Zhang D, Zhang L, Shi L, et al. In situ supported MNO-CeOx on carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3[J]. Nanoscale, 2013, 5(3): 1127–1136.
DOI:10.1039/c2nr33006g |
| [92] |
Sinfelt J H, Yates D J C. Catalytic hydrogenolysis of ethane over the noble metals of Group Ⅷ[J]. J Catal, 1967, 8(1): 82–90.
|
| [93] |
Huang Z, Zhu Z, Liu Z. Combined effect of H2O and SO2 on V2O5/AC catalysts for NO reduction with ammonia at lower temperatures[J]. Appl Catal B:Environ, 2002, 39(4): 361–368.
DOI:10.1016/S0926-3373(02)00122-4 |
| [94] |
Gao M, Zhu L, Ong W L, et al. Structural design of TiO2-based photocatalyst for H2 production and degradation applications[J]. Catal Sci Technol, 2015, 5(10): 4703–4726.
DOI:10.1039/C5CY00879D |
| [95] |
Wang M, Ioccozia J, Sun L, et al. Inorganic-modified semiconductor TiO2 nanotube arrays for photocatalysis[J]. Energy Environ Sci, 2014, 7(7): 2182–2202.
DOI:10.1039/C4EE00147H |
| [96] |
Xu N, Hu L, Zhang Q, et al. Significantly enhanced dielectric performance of poly(vinylidene fluoride-co-hexafluoropylene)-based composites filled with hierarchical flower-like TiO2 particles[J]. ACS Appl Mater Inter, 2015, 7(49): 27373–27381.
DOI:10.1021/acsami.5b08987 |
| [97] |
Wu G, Wang J, Thomas D F, et al. Synthesis of F-doped flower-like TiO2 nanostructures with high photoelectron chemical activity[J]. Langmuir, 2008, 24(7): 3503–3509.
DOI:10.1021/la703098g |
| [98] |
Zhou W, Liu X, Cui J, et al. Control synthesis of rutile TiO2 microspheres, nanoflowers, nanotrees and nanobelts via acid-hydrothermal method and their optical properties[J]. Cryst Eng Comm, 2011, 13(14): 4557–4563.
DOI:10.1039/c1ce05186e |
| [99] |
Zhao X, Huang L, Namuangruk S, et al. Morphology-dependent performance of Zr-CeVO4/TiO2 for selective ca-talytic reduction of NO with NH3[J]. Catal Sci Technol, 2016, 6(14): 5543–5553.
DOI:10.1039/C6CY00326E |
| [100] |
Han T, Chen Y, Tian G, et al. In situ synthesis of 3D fflower-like NiMnFe mixed oxides as monolith catalysts for selective catalytic reduction of NO with NH3[J]. Sci Chin Mater, 2016, 59: 1003–1016.
DOI:10.1007/s40843-016-5126-1 |
| [101] |
Ma B, Guan P, Li Q, et al. MOF-derived fflower-like MoS2@TiO2 nanohybrids with enhanced activity for hydrogen evolution[J]. ACS Appl Mater & Inter, 2016, 8(40): 26794–26800.
|
| [102] |
Khalid N S, WanZaki W S, Ahmad M K. Growth of rutile phased titanium dioxide (TiO2) nanofflowers for HeLa cells treatment[J]. IFMBE Proce, 2015, 46: 243–246.
|
| [103] |
Wang Y, Mo Z, Zhang P, et al. Synthesis of fflower-like TiO2 microsphere/graphene composite for removal of organic dye from water[J]. Mater Design, 2016, 99: 378–438.
DOI:10.1016/j.matdes.2016.03.066 |
| [104] |
Tian G, Chen Y, Zhou W, et al. 3D hierarchical flower-like TiO2 nanostructure:Morphology control and its photocatalytic property[J]. Cryst Eng Comm, 2011, 13(8): 2994–3000.
DOI:10.1039/c0ce00851f |
| [105] |
Chen Q, Chen C, Ji H, et al. Surfactant-additive-free synthesis of 3D anatase TiO2 hierarchical architectures with enhanced photocatalytic activity[J]. RSC Adv, 2013, 3(38): 17559–17566.
DOI:10.1039/c3ra42314j |
| [106] |
Wang H, Cai K, Liu J, et al. Synthesis of nanosphere TiO2 with flower-like micro-composition and its application for the selective catalytic reduction of NO with NH3 at low temperature[J]. RSC Adv, 2016, 6(87): 84294–84308.
DOI:10.1039/C6RA19006E |
| [107] |
Zong L, Dong F, Tang Z, et al. Evolution process of TiO2 shape and effect on the catalytic performance for selective catalytic reduction of NOx with NH3[J]. Catal Surveys Asia, 2018, 22(2): 105–117.
DOI:10.1007/s10563-018-9244-7 |
| [108] |
Zong L, Dong F, Tang Z, et al. Formation process of flower-like TiO2 and effect on the catalytic performance for selective catalytic reduction of NOx with NH3[J]. Chem Eng J, 2018, 343: 500–511.
DOI:10.1016/j.cej.2018.03.035 |
| [109] |
Zhang Qiao (张巧), Li Can (李灿), Liao Shi-jun (廖世军). Crystal facet effect on semiconductor-based photocatalysts (无机半导体基光催化剂的晶面电荷分离效应的研究)[D]. Guangzhou (广州): South China University of Technology (华南理工大学), 2016.
http://cdmd.cnki.com.cn/Article/CDMD-10561-1016770210.htm |
| [110] |
Xie S, Han X, Kuang Q, et al. Solid state precursor strategy for synthesizing hollow TiO2 boxes with a high percentage of reactive 001 facets exposed[J]. Chem Commun, 2011, 47(23): 6722–6724.
DOI:10.1039/c1cc11542a |
| [111] |
Zhang J, Wang J, Zhao Z, et al. Reconstruction of the (001) surface of TiO2 nanosheets induced by the ffluorinesurfactant removal process under UV-irradiation for dye-sensitized solar cells[J]. Phys Chem Chem Phys, 2012, 14(14): 4763–4769.
DOI:10.1039/c2cp24039d |
| [112] |
Yang H, Liu G, Qiao S, et al. Solvothermal synthesis and photoreactivity of anatase TiO2 nanosheets with do-minant 001 facets[J]. J Am Chem Soc, 2009, 131(36): 4078–4083.
|
| [113] |
Liu G, Yang H, Wang X, et al. Visible light responsive nitrogen doped anatase TiO2 sheets with dominant 001 facets derived from TiN[J]. J Am Chem Soc, 2009, 131(36): 12868–12869.
DOI:10.1021/ja903463q |
| [114] |
Zhang Ran (张然), He Hong (何洪). Effects of TiO2 morphologies on the activity of selective catalytic reduction of NO over V2O5/TiO2 catalysts (载体形貌对钒钛SCR催化剂脱硝活性影响)[D]. Beijing (北京): Beijing University of Technology (北京工业大学), 2016.
http://cdmd.cnki.com.cn/Article/CDMD-10005-1016785685.htm |
| [115] |
Gong X, Selloni A. Reactivity of anatase TiO2 nanoparticles:The role of the minority (001) surface[J]. J Phys Chem B, 2005, 109(42): 19560–19562.
DOI:10.1021/jp055311g |
| [116] |
Yang H, Zen H. Control of nucleation in solution growth of anatase TiO2 on glass substrate[J]. J Phys Chem B, 2003, 107(44): 12244–12255.
DOI:10.1021/jp022155u |
| [117] |
Xie Yan (谢艳), Cheng Yi-bing (程一兵), Chen Wei (陈炜). Synthesis of "Oriented Attachment" TiO2 nanorods and their applications in highly efficient dye-sensitized solar cells (取向键接性二氧化钛纳米棒的制备及其高效染料敏化太阳能电池应用)[D]. Wuhan (武汉): Huazhong University of Science & Technology (华中科技大学), 2013.
http://d.wanfangdata.com.cn/Thesis/D411791 |
| [118] |
Deng S, Meng T, Xu B, et al. Advanced MnOx/TiO2 catalyst with preferentially exposed anatase 001 facets for low-temperature SCR of NO[J]. ACS Catal, 2016, 6(9): 5807–5815.
DOI:10.1021/acscatal.6b01121 |
| [119] |
Huang L, Zha K, Namuangruk S, et al. Romotional effect of the TiO2 (001) facets in the selective catalytic reduction of NO with NH3:In situ DRIFTS and DFT studies[J]. Catal Sci Technol, 2016, 6(9): 8516–8524.
|
| [120] |
Wang H, Cao S, Fang Z, et al. CeO2 doped anatase TiO2 with exposed (001) high energy facets and its performance in selective catalytic reduction of NO by NH3[J]. Appl Surf Sci, 2015, 330: 245–252.
DOI:10.1016/j.apsusc.2014.12.163 |
| [121] |
Zhao L, Lu J. Fabrication and application of mesoporous TiO2 film coated on Al wire by sol-gel method with EISA[J]. Appl Surf Sci, 2017, 402: 369–371.
DOI:10.1016/j.apsusc.2017.01.023 |
| [122] |
Liang X, Zhang H, Li H, et al. Enhanced self-assembly of crystalline, large-area, and periodicity tunable TiO2 nanotube arrays on various substrates[J]. ACS Appl Mater Inter, 2017, 9(7): 6265–6272.
DOI:10.1021/acsami.6b12474 |
| [123] |
Bahadur J, Sen D, Mazumder S, et al. One step fabrication of thermally stable TiO2/SiO2 nanocomposite microspheres by evaporation-induced self-assembly[J]. Langmuir, 2012, 28(31): 11343–11353.
DOI:10.1021/la3022886 |
| [124] |
Bian Z, Zhu J, Wang S, et al. Self-assembly of active Bi2O3/TiO2 visible photocatalyst with ordered mesoporous structure and highly crystallized anatase[J]. J Phys Chem C, 2008, 112(16): 6258–6262.
DOI:10.1021/jp800324t |
| [125] |
Arconada N, Castro Y, Durán A, et al. Photocatalytic oxidation of methyl ethyl ketones over sol-gel mesoporous and meso-structured TiO2 films obtained by EISA method[J]. Appl Catal B:Environ, 2011, 107(1/2): 52–58.
|
| [126] |
Sarkar A, Jeon N, Noh J, et al. Well-organized mesoporous TiO2 photoelectrodes by block copolymer-induced sol-gel assembly for inorganic-organic hybrid perovskite solar cells[J]. J Phys Chem C, 2014, 118(30): 16688–16693.
DOI:10.1021/jp412655p |
| [127] |
Zong L, Zhang G, Zhao H. One pot synthesized CeO2/WO3-TiO2 catalysts with enriched TiO2 (001) facets for selective catalytic reduction of NO with NH3 by evaporation-induced self-assembly method[J]. Chem Eng J, 2018, 354: 295–303.
DOI:10.1016/j.cej.2018.07.199 |
 2020, Vol. 34
2020, Vol. 34


