2. 常州工程职业技术学院, 江苏 常州 213164
2. Changzhou Vocational Institute of Engineering, Changzhou 213164, China
化石燃料燃烧不仅会产生烟尘等固体颗粒物类空气污染物还会产生氮氧化物(NOx)这类气体形空气污染物. NOx除了会形成酸雨、光化学烟雾、臭氧层破坏、二次形成颗粒物等对生态环境造成负面影响的现象外[1-3], 还会对人类的气管、肺泡造成极大的损害, 增加罹患肺癌的风险[4]. 因此, 找寻一种脱除尾气中NOx的方式显得尤为重要. 当前, 选择性催化还原被普遍认为是脱除大气中的NOx最为有效的方法, 其中用NH3作为还原剂的NH3-SCR已成为一种较为成熟的NOx脱除技术[5]. 但NH3本身有毒、价格高、难运输、易泄露、易腐蚀设备且NH3-SCR不适用于小型固定源及移动源[6], 这就使得该技术的应用面较小, 有必要选择一种新的还原气体代替NH3.
CH4作为还原剂具有储量大、安全可靠、成本低、易处理等优点[7], 但CH4本身分子结构稳定、键能较高[8], 达到活化状态需要较高的能量, 因此较难激活CH4还原NOx[4]. 1990年Iwamoto[9]及Held等[10]制备的催化剂实现了以甲烷作还原剂的脱硝研究, 但其脱硝效率较低. 因此, 在CH4作还原剂条件下制备出脱硝效率较高的催化剂成为研究的热点方向. 此外, 工业应用的实际尾气组分中含有水蒸气和SO2, 丝光沸石(MOR)[11-12]、镁碱沸石(FER)[13-14]、ZSM-5[15-16]等沸石分子筛类催化剂, 在含水蒸气情况下容易造成结构脱铝, 从而使得催化剂结构坍塌, 进而导致催化剂部分失活或全部失活, SO2对大多数催化剂的脱硝活性也存在毒化影响[17-18], 这就使得上述类型催化剂目前不适于实际应用. 柱撑粘土(PILC)类催化剂的结构在高温且含水蒸气的工况下几乎不受影响, 此外, PILC类催化剂还具备层间距及孔径可调整、比表面积大、表面酸丰富、便宜易得等特点[19-20], 使其成为一种优良的催化剂载体. Bahamonde等[21]制备的Al-PILC在负载Rh、Pd的情况下进行CH4-SCR实验, 于400 ℃下可分别达到61%和31%的NO脱除率. 在不同类柱撑剂制备的PILC中Ti-PILC展现出较为优越的吸附特性及NOx脱除性能[22], 且有研究表明载体Ti-PILC比Al和Zn柱撑得到的载体具备更优越的催化性能[23-24].
我们主要就如何在维持催化剂高温结构稳定性的前提下, 提升PILC类催化剂的NOx脱除活性及抗水蒸气、抗SO2毒化的能力等方面展开研究. 实验采用Ti-PILC作为载体, 分步负载活性金属Ga和Fe, 在CH4∶NO=2∶1的实验工况下, 分别研究xGa/Ti-PILC(x=7、14、21)及yFe-xGa/Ti-PILC(y=5、10、15)系列催化剂选择性还原NO的特性及抗水蒸气、抗SO2毒化的能力, 并借助相关表征手段探究其物理化学性质对催化剂脱硝性能的影响.
1 实验部分 1.1 催化剂的制备将一定量的蒙脱土加入浓度为1 mol/L且温度为60 ℃的NaCl溶液中, 经恒温磁力搅拌器搅拌12 h后静置2 h, 用无水乙醇与去离子水按1∶1配比的乙醇水溶液重复离心、洗涤沉淀物3次后, 于100 ℃烘箱中干燥后得Na化蒙脱土. 用烧杯盛装一定体积一定浓度的盐酸, 用移液管量取一定体积的TiCl4并将其缓慢滴加至处于搅拌中的盐酸中, 得到盐酸与TiCl4的混合液. 10 min后向处于搅拌中的混合液中缓慢加入一定量的去离子水, 将混合液中的H+浓度控制在0.25~1 mol/L之间, 继续搅拌12 h后静置老化3 h得柱化剂. 边搅拌边将柱化剂逐滴滴入Na化蒙脱土悬浮液中, 滴定完成后继续搅拌3 h后静置12 h, 将交换完毕的混合液经离心、洗涤至不含Cl-, 100 ℃干燥后研磨并于500 ℃焙烧2 h后得到载体Ti-PILC.
取一定量的Ti-PILC粉末添加至不同浓度的水合硝酸镓盐溶液中, 采用浸渍法负载Ga, 经离心后于120 ℃干燥、500 ℃焙烧2 h后研磨得到Ga/Ti-PILC. 将Ga/Ti-PILC添加至不同浓度的九水硝酸铁溶液中并在60 ℃水浴条件下搅拌6 h, 后经离心、洗涤, 再将固体沉淀部分在110 ℃干燥12 h后于500 ℃焙烧2 h,然后经研磨、压片、过筛, 得到大小为粒径0.7~0.5 mm的颗粒状催化剂Fe-Ga/Ti-PILC.
1.2 催化剂的表征采用型号为麦克2020的全自动比表面及孔隙度分析仪进行N2吸附-脱附测试, 利用BET方程及BJH模型分别得到催化剂的比表面积、孔容及孔径数据.
以氢气发生器(EW-301HG)作为氢气源, 将气相色谱仪与立式管式炉进行组合得到H2-TPR检测系统. 将0.1 g催化剂置于直径为8 mm的石英管中, 且上下两端用石英棉固定, 在300 ℃的N2氛围下吹扫30 min以除去催化剂表面附着的杂质气体及水分, 待立式管式炉内膛温度降至室温后通入测试气体(95%N2+5%H2), 气流流量设定为20 mL/min.
利用荷兰帕纳科公司生产的型号为XPert Pro MPD的多功能粉末衍射仪进行XRD实验, 将测试角设定为2°~90°, 扫描速率控制在2 (°)/min. 测试前需将催化剂研磨为手摸无颗粒感的粉末, 后将0.5 g粉末状催化剂置于平整光洁的平面上, 并保持厚度约为1 mm. 最后将测试所得的数据经由软件Jade6.0于谱图中分析找出物相.
采用赛默飞世尔科技型号K-Alpha X射线光电子能谱仪进行XPS测试, 测试用能量为1486.6 eV的单色化Al Kα源(Mono Al Kα). 测试前将催化剂样品研磨为粉末状, 保证测试的样品的大小满足长宽小于10且高度小于3 mm, 样品表面需用乙醇擦拭干净, 并对样品进行抽真空处理.
采用铂金埃尔默公司生产的型号为PE lambda 750的紫外分光光度计进行UV-vis测试. 测试时将0.1 g催化剂样品在波长250~1000 nm范围内进行吸光度测试.
采用PerkinElmer公司的红外光谱仪进行Py-FTIR表征测试. 将测试样品研磨为粉末状且保持干燥不含水的状态, 取10~20 mg催化剂样品压片后放置于石英池中并在500 ℃条件下处理2 h, 高温处理后降至室温开始吸附吡啶, 最后分别于150和300 ℃下真空采样获得测试光谱并结合红外分析得到催化剂表面的Brønsted酸和Lewis酸信息.
1.3 催化剂的NOx脱除性能测试将400 mg大小为粒径0.7~0.5 mm的催化剂填充于内径为8 mm的石英管中, 用石英棉上下固定进行催化剂的活性测试. 活性测试开始前, 先将石英管中的催化剂于立式管式炉内在N2氛围下升温到300 ℃并维持30 min以除去催化剂上吸附的一些非模拟烟气成分的气体及水分.
活性测试所用的模拟烟气标准组成为0.1%NO、0.2%CH4、2%O2, 以N2为平衡气; 测试抗水蒸气及抗SO2特性时分别添加5%水蒸气、0.02%SO2, 控制反应气体的流量为100 mL/min, GHSV为10 000 h-1. 将实验温度设定在100~700 ℃, 每隔50 ℃设定为一个数据记录点, 实验记录前需让反应气体在催化剂表面吸附一段时间, 待烟气分析仪(ECOM-J2KN, Germany)的气体组成示数稳定后记录下反应尾气中的NO浓度、NO2浓度、NOx浓度等数据.
催化剂脱硝反应进程中的NO还原率、CH4转化率及N2选择性的计算如下:
| $C_{N O}=\frac{\phi(N O)_{i n}-\phi\mathit{(}N O\mathit{)}_{o u t}}{\phi\mathit{(}N O\mathit{)}_{i n}} \times 100 \% $ | (1) |
| $C_{C H_\mathit{4}}=\frac{\phi \mathit{(}{CH}_4 \mathit{)}_{ {in }}-\phi \mathit{(}{CH}_4 \mathit{)}_{ {out }}}{\phi \mathit{(}{CH}_4 \mathit{)}_{ {in }}} \times 100 \% $ | (2) |
| $ S_{N_\mathit{2}}=\frac{\phi\mathit{(}N O\mathit{)}_{i n}-\phi\mathit{(}N O\mathit{)}_{o u t}-\phi \mathit{(}N_2 \mathit{)}_{o u t}-2 \phi \mathit{(}N_2 O \mathit{)}_{{ }_{o u t}}}{\phi\mathit{(}N O\mathit{)}_{i n}-\phi\mathit{(}N O\mathit{)}_{ {out }}} $ | (3) |
式中: CNO为NO转化率; CCH4为CH4转化率; SN2为N2选择性; ϕ(NO)in为进口NO浓度; ϕ(NO)out为出口NO浓度; ϕ(CH4)in为进口CH4浓度; ϕ(CH4)out为出口CH4浓度; ϕ(NO2)out为出口NO2浓度; ϕ(N2O)out为出口N2O浓度.
2 结果与讨论 2.1 NOx脱除效率图 1(a)为不同固体催化剂在0.1%NO、0.2%CH4、2%O2, N2为平衡气的实验工况下的脱硝活性测试曲线. 从图中可知在CH4作为还原剂的反应条件下, 载体Ti-PILC的SCR-NO活性很差, 在引入活性金属Fe后得到的催化剂Fe/Ti-PILC并未使得催化剂的脱硝活性得到较大的提升, 但引入Ga后得到的xGa/Ti-PILC(x=7、14、21)系列催化剂均使得催化剂的SCR-NO活性在中高温(300~500 ℃)及高温(> 500 ℃)条件下得到了较为明显的提升, 其中催化剂14Ga/Ti-PILC具有最佳的脱硝活性, 于500 ℃达到了77%的NO还原率. 这表明在载体Ti-PILC上引入活性金属Ga比引入Fe的脱硝效率提升更佳. 在14Ga/Ti-PILC的基础上引入Fe得到的yFe-14Ga/Ti-PILC(y=5、10、15)系列催化剂的脱硝活性相较于Fe/Ti-PILC也有了较大提升, 其中效率最佳者10Fe-14Ga/Ti-PILC在550 ℃下达到了61%的NO还原率.
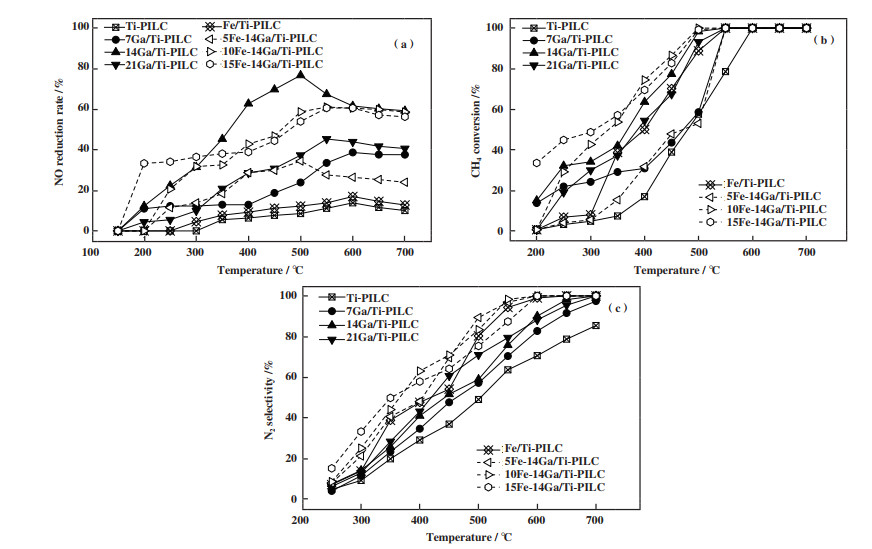
|
图 1
各固体催化剂的SCR脱硝活性
Fig.1
SCR deNOx activities over different solid catalysts
Reaction condition: ϕ(NO) = 0.1%, ϕ(CH4) = 0.2%, ϕ(O2) = 2%, N2 balance, GHSV = 10 000 h-1 (a): NOx conversion; (b): CH4 conversion; (c): N2 selectivity |
各固体催化剂的CH4转化率和N2选择性分别如图 1(b)和1(c)所示. 从中可以发现实验中的各催化剂的CH4转化率呈现随温度升高而升高的趋势, 在500 ℃下无论是仅引入活性金属Ga的xGa/Ti-PILC(x=7、14、21)系列催化剂还是同时引入活性金属Fe、Ga的yFe-14Ga/Ti-PILC(y=5、10、15)系列催化剂的CH4转化率均较载体Ti-PILC的转化率有了提升, 且含Fe的催化剂比仅含Ga的效果更好. 当反应温度达到550 ℃时, 除载体Ti-PILC外, 其他各催化剂的CH4转化率均达到了100%, REN和FIERRO等[25-26]认为适量添加Fe有利于CH4的转化, 可能是这些催化剂在接近CH4的情况下促进了CH4的氧化燃烧反应. yFe-14Ga/Ti-PILC(y=5、10、15)系列催化剂的脱硝效率虽不如14Ga/Ti-PILC, 但是其N2选择性要高于14Ga/Ti-PILC和7Ga/Ti-PILC, 这表明Fe的引入能够在一定程度上提高催化剂的N2选择性[27].
2.2 水蒸气和SO2对催化剂脱硝活性的影响燃料经燃烧后会产生一定量的水蒸气和SO2, 不少研究表明水蒸气和SO2的存在对不同催化剂的脱硝活性会造成不同程度的负面影响[28-29]. 为研究水蒸气和SO2对催化剂14Ga/Ti-PILC在CH4-SCR反应中脱硝活性的影响及Fe的引入对催化剂抗水蒸气和抗SO2性能的提升, 选取引入Fe后脱硝效率最佳的10Fe-14Ga/Ti-PILC为对比对象.
由图 2(a)可知, 于500 ℃下通入5%的水蒸气后催化剂14Ga/Ti-PILC的脱硝效率从72%开始下降且稳定在40%左右. 断除水蒸气的供给后催化剂的脱硝效率开始逐步攀升到48%. 造成这一结果的原因可能是: 水蒸气吸附在催化剂的表面占据了NO、CH4等反应气体在催化剂表面的吸附位点[30]; 也可能是因为水蒸气的存在提高了CH4还原NO所需的活化能[31], 使得达到活化状态反应的气体量减少进而降低催化剂的脱硝活性. 引入Fe后催化剂10Fe-14Ga/Ti-PILC的催化活性由69%下降并稳定在61%左右, 使催化剂在含水蒸气氛围下的脱硝效率得到了提升.
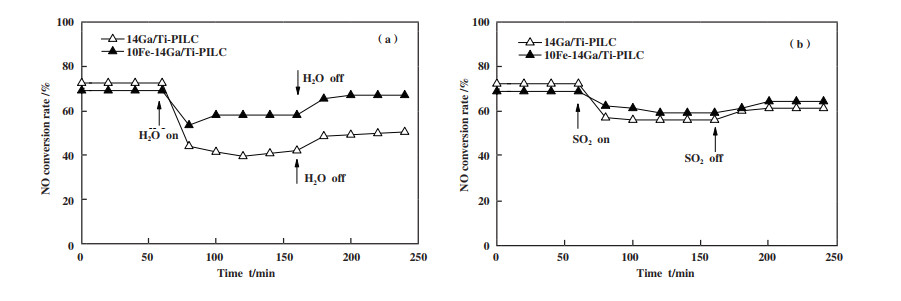
|
图 2
水蒸气和SO2对催化剂14Ga/Ti-PILC和10Fe-14Ga/Ti-PILC脱硝性能的影响
Fig.2
Effects of steam and SO2 on deNOx performance of catalysts 14Ga/Ti-PILC and 10Fe-14Ga/Ti -PILC
Reaction condition: ϕ(NO) = 0.1%, ϕ(CH4) = 0.2%, ϕ(O2) = 2%, ϕ(steam) = 5%, ϕ(SO2) = 0.02%, N2 balance, GHSV = 10 000 h-1 (a): effects of steam; (b): effects of SO2 |
由图 2(b)可知, 当通入0.02%SO2后催化剂14Ga/Ti-PILC的脱硝效率从72%下降到57%左右, 断除SO2的供给后催化剂的脱硝效率开始逐步攀升至61%左右. 在含SO2的反应氛围中14Ga/Ti-PILC的活性受SO2抑制较为明显, 但当SO2撤除后催化剂的活性有小幅的回升. 出现这种结果可能是因为SO2使得催化剂表面生成了硫酸盐物种, 而硫酸盐物种堵塞了催化剂的孔隙, 减少了催化剂的吸附表面积及活性位点的数量, 从而导致催化剂活性降低[32]; 也可能是SO2抑制了中间产物亚硝酸盐和硝酸盐的形成并产生了碳沉积物堵塞了催化剂的部分孔洞[33]. 引入Fe后能一定程度降低SO2对催化剂的毒化影响, 可将催化剂脱硝活性下降幅度控制在8%以内.
2.3 催化剂的N2吸附-脱附表征结果通常在保证结构稳定的条件下, 催化剂的比表面积越大越好. 由李前程、黄莎莎等[34-35]的研究可知, 蒙脱土(比表面积24 m2·g-1)在经Fe和Al柱撑后得到的Fe-PILC和Al-PILC分别可使比表面积达到194.7和86 m2·g-1, 本研究中使用Ti柱撑后得到的载体Ti-PILC比使用Fe、Al柱撑后得到的载体表面积(205 m2·g-1)更大. 在Ti-PILC上负载活性金属Ga后得到的催化剂xGa/Ti-PILC(x=7、14、21)的孔容与孔径变化幅度均较小, 这也与Yogo等[36]引入Ga后结果相一致. 引入Ga后的xGa/Ti-PILC的孔径较Ti-PILC有些微下降但孔容得到了提升且孔容随着Ga的负载量呈现先增加后减小的趋势, 这可能是Ga的负载量超过一定量后形成的Ga2O3颗粒堵塞了部分孔洞, 且会影响到催化剂对NO的脱除效率, 这不仅与xGa/Ti-PILC的脱硝效率相吻合也与Shimizu等[37]的推测相一致. 当Ti-PILC负载较多的Fe后, 催化剂的表面会形成质地多孔的FexOy从而使得催化剂的比表面积得到增加, 但可能造成催化剂孔洞的堵塞[38], 这可能是Fe/Ti-PILC虽比表面积较大但孔容较小的原因.
对照IUPAC给出的分类标准可知, 图 3(a)中所测试的各固体催化剂的吸附脱附等温线均属于Ⅳ型等温线, 所对应的孔径为介孔(2~50 nm). 在相对压力p/p0 < 0.1的区段等温线呈现出轻微上凸的形式, 这主要是单分子层的吸附造成的, 表明催化剂内部很可能存在部分微孔结构; 0.4 < p/p0区段出现较为明显的H3型回滞环[39], 一般是因为发生毛细管凝聚所产生且所形成的回滞环, 其形状受催化剂内部孔的形状及大小影响.
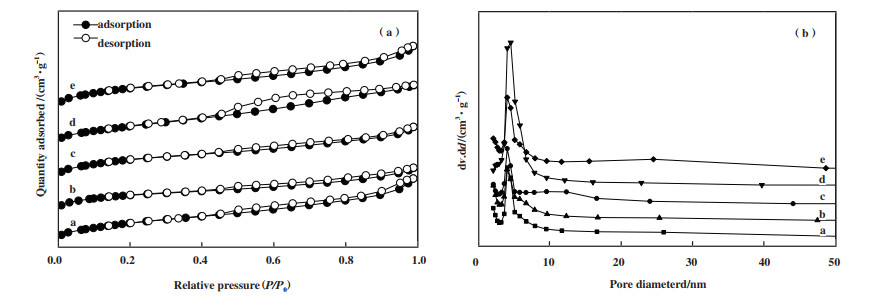
|
图 3 各固体催化剂的N2吸附-脱附等温曲线和孔径分布 Fig.3 N2 adsorption-desorption isothermal curve and pore size distribution of solid catalysts a: Ti-PILC; b: Fe/Ti-PILC; c: 14Ga/Ti-PILC; d: 10Fe-14Ga/Ti-PILC; e: 15Fe-14Ga/Ti-PILC |
由图 3(b)可知, 在14Ga/Ti-PILC上引入Fe后使催化剂的孔径部分变大且孔径分布朝着更宽的区域, 这很可能是在催化剂表面形成的多孔的FexOy物质造成的. 但是有相关研究表明, 在含活性金属Ga的催化剂中水蒸气对孔径较大的催化剂的脱硝活性影响较低[40]. 结合14Ga/Ti-PILC和10Fe-14Ga/Ti-PILC在含水蒸气工况下的脱硝活性并结合这两种催化剂的孔径分布, 推测在介孔催化剂Ga/Ti-PILC上引入Fe后增大了催化剂的孔径, 而大孔径降低了水蒸气经过时在其表面的滞留时间, 进而削弱水蒸气的竞争吸附能力, 从而减小了水蒸气对催化剂表面Ga活性中心的影响, 提升了催化剂的抗水蒸气影响能力. 且通过对比催化剂10Fe-14Ga/Ti-PILC在500 ℃下进行抗水蒸气、抗SO2活性测试前后的表面织构特性数据发现, 10Fe-14Ga/Ti-PILC的孔径、孔容、比表面积近乎未发生变化, 这表明催化剂的结构并未发生变化, 即未发生结构坍塌.
2.4 催化剂的XRD表征结果图 4(a)为蒙脱土K-10(original clay)和经Ti柱撑后的载体TI-PILC的XRD谱图, 图 4(b)为各参与脱硝活性测试的固体催化剂的XRD谱图. 图 4(a)中在9°附近出现了较为明显的蒙脱土的晶面间距d(001)特征峰[41-42], 在35°附近出现了归属于层状粘土二维(hk)晶面衍射峰[22, 42]. 对比蒙脱土K-10的XRD分析TI-PILC可发现, 经Ti柱撑后得到的载体Ti-PILC的d(001)特征峰移动到了6.4°处, 也能较为明显地观察到层状粘土的hk晶面衍射峰. 该柱撑过程较好地保持了原粘土的层状结构, 且较为良好地撑开了粘土的层状结构使得其比表面积由24增大到205 m2·g-1. 在衍射角2θ为25.7°、28.2°和48.0°处出现了属于Ti的特征峰.
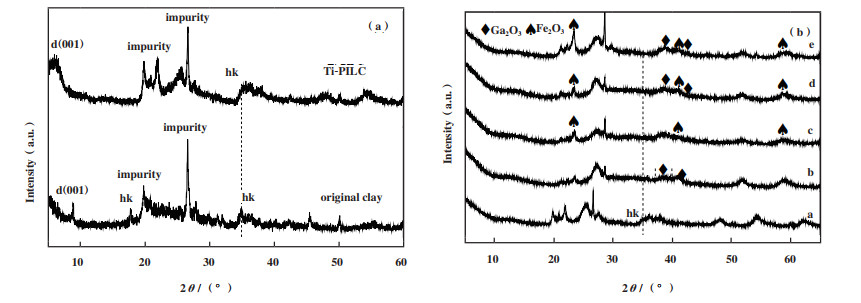
|
图 4 各固体催化剂XRD谱图 Fig.4 The XRD spectra of solid catalysts a: Ti-PILC; b: 14Ga/Ti-PILC; c: Fe/Ti-PILC; d: 10Fe-14Ga/Ti-PILC; e: 15Fe-14Ga/Ti-PILC |
分析图 4(b)的衍射图谱可发现, 含Ga的固体催化剂在35.5°和39.1°处出现了属于Ga2O3的特征峰, 且14Ga/Ti-PILC相比于10Fe-14Ga/Ti-PILC和15Fe-14Ga/Ti-PILC在35.8°处的峰形更宽, 这表明14Ga/Ti-PILC上负载的Ga物种以小的粒径形态高度分散在催化剂的表面, 这可能是14Ga/Ti-PILC脱硝活性高的重要原因. 含Fe的固体催化剂在23.4°、37.8°以及54.5°处存在Fe2O3的特征峰, 且随着Fe负载量的增加Ga的特征峰开始逐渐变小, 即Ga的含量减少, 这可能与yFe-14Ga/Ti-PILC催化剂的脱硝活性降低有一定的相关性.
2.5 催化剂的H2-TPR表征结果在进行SCR-NO反应时催化剂本身所具有的还原能力对其脱硝效率有着较大的影响[43]. 经表征测试得到实验各固体催化剂的H2 -TPR谱图如图 5所示. 其中14Ga/Ti-PILC仅在550 ℃出现还原峰, 这可能是由Ga3+→Ga+的还原形成的[44-45]. 10Fe-14Ga/Ti-PILC、15Fe-14Ga/Ti-PILC和Fe/Ti-PILC在335 ℃附近均出现了还原峰, Lin等[43]认为这一还原峰可能是由催化剂表面的FexOy中的Fe3+还原为Fe2+所形成的. Ren[25]和Fierro [26]等认为向催化剂中添加一定量Fe有利于CH4的转化, 这也与前文中含Fe催化剂的CH4转化率得到提升相契合; KOBAYASHI等认为Fe2O3能够促进CH4的燃烧, 从而使得参与SCR-NO反应的CH4含量减少, 降低了催化剂的脱硝活性, 这也能解释在14Ga/Ti-PILC的基础上引入Fe后各含Fe的催化剂的脱硝效率均有一定程度的下降. 此外, 10Fe-14Ga/Ti-PILC和15Fe-14Ga/Ti-PILC于585和685 ℃也出现了还原峰, 585 ℃处的还原峰可能是引入Fe后, 降低了Ga2O3的可还原性, 导致其从550位移至585 ℃; 而685 ℃处的还原峰可能是由Fe3O4还原为Fe0而形成[46]. 可能正是因为500 ℃后10Fe-14Ga/Ti-PILC和15Fe-14Ga/Ti-PILC仍存在难以被还原的Fe物质才使得这两种催化剂在500 ℃后脱硝效率下降幅度较低.
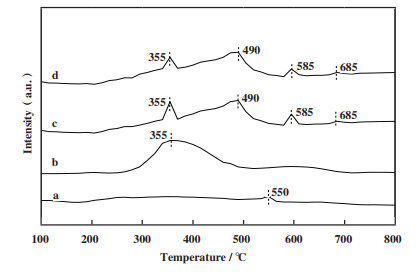
|
图 5 各固体催化剂的H2-TPR谱图 Fig.5 The H2-TPR spectra of solid catalysts a: 14Ga/Ti-PILC; b: Fe/Ti-PILC; c: 15Fe-14Ga/Ti-PILC; d: 10Fe-14Ga/Ti-PILC |
图 6(a)为Ga 2p的谱图, 由图可知催化剂14Ga/Ti-PILC的峰面积比另外两种催化剂的大, 这表明14Ga/Ti-PILC的表面含有更多的活性金属Ga. 在结合能为1118和1145.5 eV附近出现的2个特征峰, 分别归属于Ga3+的Ga 2p3/2和Ga 2p1/2自旋轨道[47]. 高度分散的活性金属Ga以Ga3+离子形式占据催化剂表面, 使催化剂具有更高的甲烷催化还原活性[48], 引入铁后, Ga3+的含量仍占主导地位但减少了Ga3+在催化剂表面的含量, 这也与活性测试中14Ga/Ti-PILC引入Fe后所展现出的NO还原率下降相一致. 且引入过多铁后, 15Fe-14Ga/Ti-PILC催化剂Ga 2p谱图分布不对称, 表明Ga3+可能存在其他结构, 因此, 引入过量铁会影响Ga3+的四面体位置分布, 影响甲烷催化还原活性, 这与XRD的分析结论相一致.
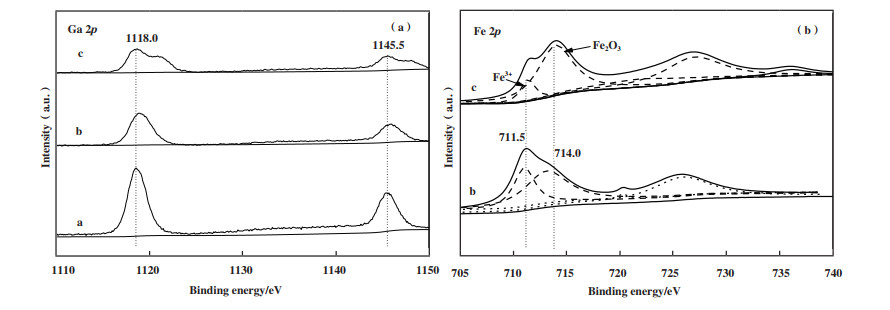
|
图 6
各固体催化剂相关元素的XPS谱图
Fig.6
XPS spectra of relevant elements of solid catalysts
(a): Ga 2p; (b): Fe 2p a: 14Ga/Ti-PILC; b: 10Fe-14Ga/Ti-PILC; c: 15Fe-14Ga/Ti-PILC |
由图 6(b)可知, 引入Fe后在711.5和714.0 eV附近出现的特征峰为Fe 2p1/2峰和Fe 2p3/2及其相应卫星峰, 分别归属于Fe3+和Fe2O3物种[49]. 其中10Fe-14Ga/Ti-PILC与Fe修饰的Ga2O3-Al2O3相似, 催化剂表面主要以游离态Fe3+存在, 增加Fe的负载量后15Fe-14Ga/Ti-PILC中的Fe2O3含量上升. 有研究表明富氧条件下Fe2O3颗粒物种的存在会促进甲烷参与氧化燃烧反应[50], 从而导致CH4选择性还原NO的反应减弱, 也与图 1(a)和图 1(b)中10Fe-14Ga/Ti-PILC比15Fe-14Ga/Ti-PILC具有更高的CH4转化率的同时, 却具有更低的NO转化率的实验结果吻合.
2.7 催化剂的UV-vis表征结果紫外可见近红外光谱可通过催化剂的光学吸收性能得到其相关元素的组分及配位结构信息. 通常, Ga2O3的吸收峰于 < 250 nm波段; 200~300 nm波长处的吸收峰归属于游离态的+3价Fe离子[22, 34, 51]; 300~400 nm处为FexOy团聚物的吸收峰[43]; 大于400 nm波段的吸收峰为固体催化剂表面形成的Fe2O3颗粒物[43, 52]. 由图 7可知, 随着活性金属负载量的增加, 各固体催化剂a-b的最大吸收强度相继增强且发生红移.
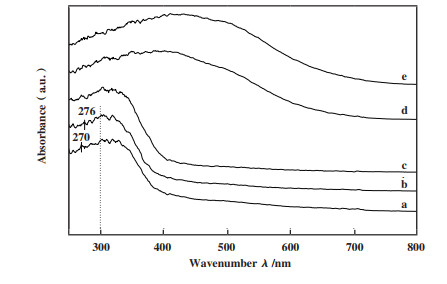
|
图 7 各固体催化剂的UV-vis谱图 Fig.7 UV-vis spectra of relevant elements of solid catalysts a: 15Fe-14Ga/Ti-PILC; b: 10Fe-14Ga/Ti-PILC; c: 15Fe/Ti-PILC; d: 14Ga/Ti-PILC; e: Ti-PILC |
其中, 10Fe-14Ga/Ti-PILC在276 nm和15Fe-14Ga/Ti-PILC于270 nm处出现的吸收峰可能是位于蒙脱土中的Al2O3物质上的Fe元素发生的t1→t2或t1→e电荷转移而形成的孤立八面体配位形式的Fe3+[53]. 含Fe的3组催化剂在300~400 nm波段的吸收峰的强度要显著高于Ti-PILC及14Ga/Ti-PILC, 这表明300~400 nm的含铁催化剂含有更高含量的FexOy团聚物, 而相关研究表明块状或大颗粒的FexOy更容易促进碳氢化合物燃烧等副反应[26, 54], 这就使得参与SCR-NOx反应的CH4的量降低, 也使得含铁催化剂的脱硝效率低于14Ga/Ti-PILC. 此外, 有研究表明游离态Fe3+低温能促进甲烷活化成脱硝反应的重要中间体HCHO[50], 而在250~300 nm波段10Fe-14Ga/Ti-PILC比15Fe-14Ga/Ti-PILC及15Fe/Ti-PILC含有更多与Fe3+的吸收峰, 这可能与10Fe-14Ga/Ti-PILC较其他两者活性高相关. 这些均与各固体催化剂的脱硝活性测试相契合.
2.8 催化剂的Py-FTIR表征结果Huang等[55]认为酸性位点是反应中间体转化为氮的关键, Wang等[56]认为催化剂的Lewis酸(L酸)和Brønsted酸(B酸)位点是决定CH4还原NO的重要参数. 为定性且定量分析各种固体催化剂表面的L酸和B酸, 将实验所用样品Ti-PILC、14Ga/Ti-PILC、Fe/Ti-PILC、10Fe-14Ga/Ti-PILC、15Fe-14Ga/Ti-PILC分别于150和300 ℃下进行Py-FTIR表征测试. 所得的L酸与B酸的实验数据如图 8和表 2所示.
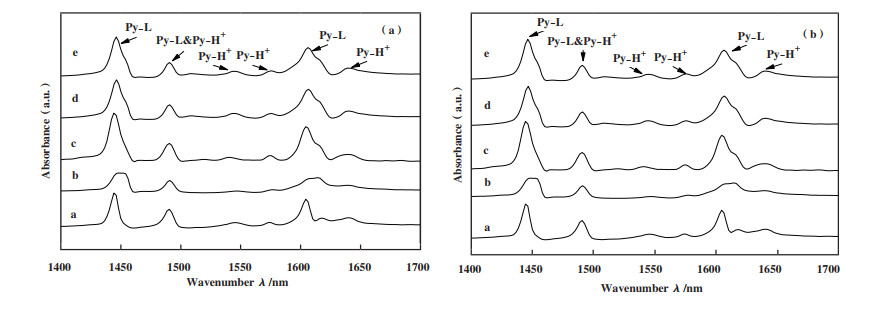
|
图 8
各固体催化剂的Py-IR光谱谱图
Fig.8
Py-IR spectra of solid catalysts
(a): 150℃ desorption; (b): 300℃ desorption a: Ti-PILC; b: 14Ga/Ti-PILC; c: Fe/Ti-PILC; d: 10Fe-14Ga/Ti-PILC; e: 15Fe-14Ga/Ti-PILC |
| 表 1 蒙脱土及各固体催化剂的织构特性 Table 1 Textual properties of montmorillonite and solid catalysts |
| 表 2 各固体催化剂的L酸和B酸含量 Table 2 Lewis and Brønsted acid content of different solid catalysts |
从图 8中可知用于实验的各固体催化剂在1446、1488、1547、1575、1607及1640 cm-1附近出现了吸收峰. 通常于1440~1460和1600~1635 cm-1波段出现的吸收峰为Py与L酸中心形成的PyL吸收峰, 1535~1550 cm-1波段为Py与B酸中心形成的PyH+吸收峰[57], 1490 cm-1附近的吸收峰由Py与L酸和B酸共同作用形成[22, 58]. 因此1446和1607 cm-1以及1547、1575和1640 cm-1处出现的吸收峰归属于B酸, 1448 cm-1处的吸收峰属于L酸和B酸的共同峰.
由表 2可知, Na化蒙脱土在经TiCl4柱撑后可使载体(Ti-PILC)表面出现B酸, 但通过分析所测试的各固体催化剂所展现出的脱硝活性可以发现: 经柱撑后催化剂的L酸含量有较小幅度的增加; 在B酸含非0的条件下, B酸的含量明显低于L酸且各催化剂的SCR-NO的活性与B酸和L酸的含量分别呈现负相关和正相关的关系. 这可能是因为L酸能够促进还原剂CH4达到活化态进而以反应物的状态参与到SCR-NO反应中[7]. 结合前文中各催化剂的脱硝活性推测所制备的各固体催化剂的活性中心很可能位于L酸位点, 即L酸含量越高, PILC系催化剂在CH4作还原剂的SCR-NO反应中活性越高.
3 结论以Ti-PILC为载体可实现在较大的比表面积和孔容时避免催化剂在高温含水蒸气工况下结构坍塌现象的发生; Ti-PILC负载活性金属Ga可有效提升PILC系列催化剂在CH4做还原剂的SCR-NO反应中的活性, 其中14Ga/Ti-PILC在500 ℃可实现77%的NOx脱除效率. 但Ga/Ti-PILC的NOx脱除效率受水蒸气影响较大, 通过引入适量的活性金属Fe增加催化剂的孔径而减少水蒸气、SO2在催化剂表面的滞留时间, 可有效抑制因水蒸气造成的催化剂活性降低并提升催化剂抗SO2毒化的能力, 且Fe的引入可提升CH4在500 ℃下的转化率. 本研究中所制备的PILC系列催化剂的脱硝活性与催化剂表面Lewis酸的含量呈正相关关系.
| [1] |
Campa M C, Doyle A M, Fierro G, et al. Simultaneous abatement of NO and N2O with CH4 over modified Al2O3 supported Pt, Pd, Rh[J]. Catal Today, 2022, 384: 76–87.
|
| [2] |
Xu Y, Wang X, Qin M, et al. Selective catalytic reduction of NOx with CH4 over zeolite catalysts: Research progress, challenges and perspectives[J]. J Environ Chem Eng, 2022, 10(2): 1–10.
|
| [3] |
Wen N, Lin R, Su Y, et al. SCR of NO with CH4 over Fe/Ga2O3-Al2O3 and the mechanism[J]. J Environ Chem Eng, 2021, 9(1): 1–10.
|
| [4] |
a. Zhao J, Zhang G, He J, et al. Effect of preparation and reaction conditions on the performance of In/H-Beta for selective catalytic reduction of NOx with CH4[J]. Chemosphere, 2020, 252: 1-12. b. Ding Xin(丁鑫), Li Guo-bo(李国波), Huang Jun(黄俊), et al. Study on denitration reaction mechanism of Pd modified multiactive site catalyst NH3-SCR(Pd改性多活性位点催化剂NH3-SCR脱硝反应机理研究)[J]. J Mol Catal(China)(分子催化), 2022, 36(1): 1-11. c. Xie Wang-wang(谢旺旺), Zhou Guang-he(周广贺), ZhangXiao-hong(张晓虹), et al. Research progress of attapulgiteapplication in flue gas SCR denitration catalytic reaction(凹凸棒石在烟气SCR脱硝催化反应中的应用研究进展)[J]. J Mol Catal(China)(分子催化), 2020, 34(6): 546-558. |
| [5] |
a. Arfaoui J, Ghorbel A, Petitto C, et al. Novel V2O5-CeO2-TiO2-SO42- nanostructured aerogel catalyst for the low temperature selective catalytic reduction of NO by NH3 in excess O2[J]. Appl Catal B, 2018, 224: 264-275. b. Li Ke-zhi(李柯志), Luo He(罗河), Zhao Ran(赵冉), et al. Research on the influence of calcined titanium dioxideto the newly-produced selective catalytic reduction catalyst and the mechanism(具焙烧服役史钛白粉对新制脱硝催化剂活性影响机制研究)[J]. J Mol Catal(China)(分子催化), 2020, 34(5): 415-424. c. Qiao Ming(乔明), Zhang Ji-yi(张继义), Zong Lu-yao(宗路遥), et al. Research progress in catalytic denitrifica-tion performance: The catalyst type, preparation methods and activity(催化脱硝技术研究进展—催化剂的种类、制备方法及催化活性)[J]. J Mol Catal(China)(分子催化), 2020, 34(2): 165-181. |
| [6] |
Maunula T, Ahola J, Hamada H. Reaction mechanism and kinetics of NOx reduction by methane on In/ZSM-5 under lean conditions[J]. Appl Catal B, 2006, 64(1): 13–24.
|
| [7] |
Zhang H Y, Li N, Li L, et al. Selective catalytic reduction of NO with CH4 over In-Fe/Sulfated zirconia catalysts[J]. Catal Lett, 2011, 141(10): 1491–1497.
DOI:10.1007/s10562-011-0681-4 |
| [8] |
Teng Z, Zhang H, Huang S, et al. Experimental study on reduction of NO by CH4 over La0.8Sr0.2MnO3/α-Al2O3 in excess of O2[J]. J Taiwan Inst Chem E, 2018, 87: 204–210.
DOI:10.1016/j.jtice.2018.03.036 |
| [9] |
Iwamoto M. Selective reduction of NO by lower hydroc-arbons in the presence of O2 and SO2 over cupper ion-exchanged zeolites[J]. Shokubai, 1990, 32: 430–433.
|
| [10] |
Held W, Konig A, Richter T, et al. Catalytic NOxredu-ction in net oxidizing exhaust gas[J]. SAE Trans, 1990, 99: 209–216.
|
| [11] |
Mendes A N, Lauga V, Capela S, et al. Application of PdCe-HMOR catalyst as NOx CH4-SCR system for heavy-duty vehicles moved by natural gas[J]. Top Catal, 2016, 59(10): 982–986.
|
| [12] |
Cano M, Guarín F, Aristizábal B, et al. Catalytic activity and stability of Pd/Co catalysts in simultaneous selective catalytic reduction of NOx with methane and oxidation of o-dichlorobenzene[J]. Catal Today, 2017, 296: 105–117.
DOI:10.1016/j.cattod.2017.05.049 |
| [13] |
Lim J B, Shin J, Ahn N H, et al. Selective catalytic reduction of NO with CH4 over cobalt-exchanged cage-based, small-pore zeolites with different framework structures[J]. Appl Catal B, 2020, 267: 1–9.
|
| [14] |
Ferreira A P, Capela S, Da Costa P, et al. CH4-SCR of NO over Co and Pd ferrierite catalysts: Effect of preparation on catalytic performance[J]. Catal Today, 2007, 119(1): 156–165.
|
| [15] |
Chen S, Yan X, Chen J, et al. Selective catalytic reduc-tion of NO in excess oxygen by methane over Mn/ZSM-5 catalysts[J]. Chin J Catal, 2010, 31(9): 1107–1114.
|
| [16] |
Li Y, Battavio P J, Armor J N. Effect of water vapor on theselective reduction of NO by methane over cobaltexcha-nged ZSM-5[J]. J Catal, 1993, 142(2): 561–571.
DOI:10.1006/jcat.1993.1231 |
| [17] |
Li Y, Armor J N. The effect of SO2 on the catalytic performance of Co-ZSM-5 and Co-ferrierite for the selective reduction of NO by CH4 in the presence of O2[J]. Appl Catal B, 1995, 5(4): 257–270.
DOI:10.1016/0926-3373(95)00007-0 |
| [18] |
Lónyi F, Solt H E, Valyon J, et al. The activation of NO and CH4 for NO-SCR reaction over In- and Co-containing H-ZSM-5 catalysts[J]. J Mol Catal A-Chem, 2011, 345(1): 75–80.
|
| [19] |
Cardona Y, Korili S A, Gil A. Understanding the formationof Al13 and Al30 polycations to the development of microporous materials based on Al13-and Al30-PILC montmorillonites: A review[J]. Appl Clay Sci, 2021, 203: 1–16.
|
| [20] |
Asgari M, Vitale G, Sundararaj U. Synthesis and characteri-zation of a novel nickel pillared-clay catalyst: In-situ carbon nanotube-clay hybrid nanofiller from Ni-PILC[J]. Appl Clay Sci, 2021, 205: 1–10.
|
| [21] |
Bahamonde A, Mohino F, Rebollar M, et al. Pillared clay and zirconia-based monolithic catalysts for selective catalytic reduction of nitric oxide by methane[J]. Catal Today, 2001, 69(1): 233–239.
|
| [22] |
Dong Shi-lin(董士林), Su Ya-xin(苏亚欣), Liu Xin(刘欣), et al. Study on Fe/Ti-PILC for selective catalytic reduction of NO by C3H6(Fe/Ti-PILC用于C3H6选择性催化还原NO的研究)[J]. J Fuel Chem Technol(燃料化学学报), 2018, 46(10): 1231–1239.
|
| [23] |
Chmielarz L, Kustrowski P, Zbroja M, et al. SCR of NO by NH3 on alumina or titania-pillared montmorillonite various modified with Cu or Co: Part I. General characterization and catalysts screening[J]. Appl Catal B, 2003, 45(2): 103–116.
DOI:10.1016/S0926-3373(03)00121-8 |
| [24] |
Wen N, Dong S, Su Y, et al. Effect of synthesis param-eters on catalytic performance of Fe/Ti-PILC catalysts for SCR-C3H6 and in situ DRIFTS study[J]. J Environ Chem Eng, 2020, 8(6): 1–8.
|
| [25] |
Ren L L, Zhang T. Reduction of NO with methane over Fe/ZSM-5 catalysts[J]. Chin Chem Lett, 2010, 21(6): 674–677.
DOI:10.1016/j.cclet.2010.02.001 |
| [26] |
Fierro G, Moretti G, Ferraris G, et al. A Mossbauer and structural investigation of Fe-ZSM-5 catalysts: Influence of Fe oxide nanoparticles size on the catalytic behaviour for the NO-SCR by C3H8[J]. Appl Catal B, 2011, 102(1): 215–223.
|
| [27] |
Wang X, Zhang T, Sun X, et al. Enhanced activity of an In-Fe2O3/H-ZSM-5 catalyst for NO reduction with methane[J]. Appl Catal B, 2000, 24(3): 169–173.
|
| [28] |
Pan H, Jian Y F, Yu Y K, et al. Regeneration and sulfurpoisoning behavior of In/H-BEA catalyst for NOx reduc-tion by CH4[J]. Appl Surf Sci, 2017, 401: 120–126.
|
| [29] |
Li Y X, Wang Q H, Wang D, et al. NO-CH4-SCR over core-shell MnH-zeolite composites[J]. Appl Sci-Basel, 2019, 9(9): 1–12.
|
| [30] |
Takahashi M, Nakatani T, Iwamoto S, et al. Effect of the composition of spinel-type Ga2O3-Al2O3-ZnO catalysts on activity for the CH4-SCR of NO and optimization of catalyst composition[J]. Ind Eng Chem Res, 2006, 45(10): 3678–3683.
|
| [31] |
Li Y, Armor J N. Selective reduction of NOx by methane on Co-Ferrierites. I. Reaction and kinetic studies[J]. J Catal, 1994, 150(2): 376–387.
|
| [32] |
Ohtsuka H, Tabata T, Hirano T. Palladium–platinum-loaded sulfated zirconia: A highly durable catalyst for the reduction of nitrogen oxides by methane in the presence of water vapor and SOx[J]. Appl Catal B, 2000, 28(2): 73–76.
|
| [33] |
Decyk P, Kim D K, Woo S I. Poisoning effect of SO2 on NO reduction by i-Butane over Fe/ZSM-5 prepared by sublimation method[J]. J Catal, 2001, 203(2): 369–374.
|
| [34] |
Li Qian-cheng(李前程), Su Ya-xin(苏亚欣), Dong Shi-lin(董士林), et al. Selective reduction of NO by propy-lene catalyzed by Fe-PILC under lean burn conditions(Fe-PILC在贫燃条件下催化丙烯选择性还原NO)[J]. J Fuel Chem Technol(燃料化学学报), 2018, 46(10): 1240–1248.
|
| [35] |
Huanng Sha-sha(黄莎莎), Su Ya-xin(苏亚欣), Zhou Xia(周夏), et al. CH4-SCR denitration performance of bimeta-llic catalyst Fe-Co/Al-PILC(双金属催化剂Fe-Co/Al-PILC的CH4-SCR脱硝性能)[J]. J Chem Eng Chin Univer(高校化学工程学报), 2021, 35(6): 1073–1081.
|
| [36] |
Yogo K, Tanaka S, Ihara M, et al. Selective reduction of NO with propane on gallium ion-exchanged zeolites[J]. Chem Lett, 1992, 1992(6): 1025–1028.
|
| [37] |
Shimizu K I, Satsuma A, Hattori T. Selective catalytic reduction of NO by hydrocarbons on Ga2O3/Al2O3 cataly-sts[J]. Appl Catal B, 1998, 16(4): 319–326.
|
| [38] |
Valverde J L, De Lucas A, Sánchez P, et al. Cation exchanged and impregnated Ti-pillared clays for selective catalytic reduction of NOx by propylene[J]. Appl Catal B, 2003, 43(1): 43–56.
|
| [39] |
Sing K S W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity[J]. Pure Appl Chem, 1985, 57(4): 603–619.
|
| [40] |
Masuda T, Watanabe T, Miyahara Y, et al. Synthesis of Ga2O3-Al2O3 catalysts by a coprecipitation method for CH4-SCR of NO[J]. Top Catal, 2009, 52(6): 699.
|
| [41] |
Gonzalez-Rodríguez B, Trujillano R, Rives V, et al. Stru-ctural, textural and acidic properties of Cu-, Fe- and Cr-doped Ti-pillared montmorillonites[J]. Appl Clay Sci, 2015, 118: 124–130.
|
| [42] |
Chmielarz L, Piwowarska Z, Kustrowski P, et al. Comp-arison study of titania pillared interlayered clays and porous clay heterostructures modified with copper and iron as catalysts of the DeNOx process[J]. Appl Clay Sci, 2011, 53(2): 164–173.
|
| [43] |
Lin Rui(林睿), Su Ya-xin(苏亚欣), Cheng Jiang-hao(程江浩), et al. Catalytic performance of Fe/Ga2O3-Al2O3 for methane reduction of NO(Fe/Ga2O3-Al2O3催化甲烷还原NO的性能)[J]. Chin J Environ Eng(环境工程学报), 2020, 14(6): 1592–1604.
|
| [44] |
Shao C T, Lang W Z, Yan X, et al. Catalytic performance of gallium oxide based-catalysts for the propane dehydrogenation reaction: Effects of support and loading amount[J]. Royal Soc Open Sci, 2017, 7(8): 4710–4723.
|
| [45] |
El-Malki E M, Van Santen R A, Sachtler W M H. Introduction of Zn Ga and Fe into HZSM-5 cavities by sublimation: Identification of acid sites[J]. J Phys Chem B, 1999, 103(22): 4611–4622.
|
| [46] |
Long R Q, Yang R T. Selective catalytic reduction of NO with ammonia over Fe3+ exchanged mordenite (Fe-MOR): Catalytic performance, characterization and mechanistic study[J]. J Catal, 2002, 207(2): 274–285.
|
| [47] |
Gao Jun-hua(高俊华), Liu Ping(刘平), Ji Ke-ming(吉可明), et al. Synthesis and characterization of GaZSM-5 molecular sieve and its catalytic performance in methanol to hydrocarbon (MTH) reaction(GaZSM-5分子筛的合成、表征及其在甲醇转化制烃(MTH)反应中的催化性能)[J]. J Fuel Chem Technol(燃料化学学报), 2018, 46(4): 465–472.
|
| [48] |
Miyahara Y, Watanabe T, Masuda T, et al. Evaluation of catalytic activity of Ga2O3-Al2O3 solid solutions for CH4-SCR by UV-vis spectra after adsorption of C3H6 as a probe[J]. J Catal, 2008, 259(1): 36–42.
|
| [49] |
Yang S, Guo Y, Yan N, et al. Remarkable effect of the incorporation of titanium on the catalytic activity and SO2 poisoning resistance of magnetic Mn-Fe spinel for elemental mercury capture[J]. Appl Catal B, 2011, 101(3): 698–708.
|
| [50] |
Zhang Q, Li Y, An D, et al. Catalytic behavior and kinetic features of FeOx/SBA-15 catalyst for selective oxidation of methane by oxygen[J]. Appl Catal A, 2009, 356(1): 103–111.
|
| [51] |
Pérez-RamíRez J, Santhosh Kumar M, Brückner A. Reduction of N2O with CO over FeMFI zeolites: Influence of the preparation method on the iron species and catalytic behavior[J]. J Catal, 2004, 223(1): 13–27.
|
| [52] |
Li L, Shen Q, Li J, et al. Iron-exchanged FAU zeolites: Preparation, characterization and catalytic properties for N2O decomposition[J]. Appl Catal A, 2008, 344(1): 131–141.
|
| [53] |
Kumar M S, Schwidder M, Grünert W, et al. On the natu-re of different iron sites and their catalytic role in Fe-ZSM-5 DeNOx catalysts: New insights by a combined EPR and UV/VIS spectroscopic approach[J]. J Catal, 2004, 227(2): 384–397.
|
| [54] |
Bachari K, Millet J M M, Bonville P, et al. Spectroscopic characterization of iron nanoparticles in Fe-mesoporous silicate catalysts[J]. J Catal, 2007, 249(1): 52–58.
|
| [55] |
Huang F J, Hu W, Chen J J, et al. Insight into enhanc-ement of NO reduction with methane by multifunctional catalysis over a mixture of Ce/HZSM-5 and CoOx in excess of oxygen[J]. Ind Eng Chem Res, 2018, 57(40): 13312–13317.
|
| [56] |
Wang H, Li B, Lu X B, et al. Selective catalytic reduction of NO by methane over the Co/MOR catalysts in the presence of oxygen[J]. J Fuel Chem Technol, 2015, 43(9): 1106–1112.
|
| [57] |
Datka J, Turek A M, Jehng J M, et al. Acidic properties of supported niobium oxide catalysts: An infrared spectroscopy investigation[J]. J Catal, 1992, 135(1): 186–199.
|
| [58] |
Sultana A, Haneda M, Fujitani T, et al. Influence of Al2O3 support on the activity of Ag/Al2O3 catalysts for SCR of NO with decane[J]. Catal Lett, 2007, 114(1): 96–102.
|
 2022, Vol. 36
2022, Vol. 36


