2. 昆明理工大学 挥发性有机物污染防治与资源化省创新团队, 云南 昆明 650500;
3. 云南省高校恶臭挥发性有机物控制重点实验室, 云南 昆明 650500;
4. 昆明理工大学 化学工程学院, 云南 昆明 650093
2. The Innovation Team for Volatile Organic Compounds Pollutants Control and Resource Utilization of Yunnan Province, Kunming 650500, China;
3. The Higher Educational Key Laboratory for Odorous Volatile Organic Compounds Pollutants Control of Yunnan Province, Kunming 650500, China;
4. Faculty of Chemical Engineering, Kunming University of Science and Technology, Kunming, 650093, China
苯系物, 即芳香族化合物(Monoaromatic Hydroc-arbons), 因苯(benzene)、甲苯(toluene)、乙苯(ethylbe-nzene)、二甲苯(xylene)4类为其中的代表性物质, 在总挥发性有机物(total volatile organic components, TVOCs)中占据着重要的位置, 所以简称为BTEX. BTEX来源广泛[1-2], 且不同来源的组成、特性及排放量差异较大. 释放到环境中的BTEX会在各环境介质间迁移、富集, 进而对生态和人体健康产生众多不利影响[3]. 相关研究发现, BTEX具有神经毒性[4]、免疫毒性[5]、遗传毒性[6]等危害, 人体长期接触会导致患上贫血症和白血病[7], 此外, 许多BTEX具有刺激性气味, 能产生令人不愉快的感觉, 对生态环境质量具有负面影响. 同时, BTEX会形成大气中光氧化剂(如O3[8]和PAN等)和二次有机气溶胶(Secondary organic aerosol, SOAs)等[9-10], 进而引发区域性环境问题.
当前, 苯系物的控制技术种类较多[11-13], 其中, 热催化氧化以适用范围广、能耗低、二次污染少等优点被广泛应用于VOCs的治理, 而该技术的核心是高活性、高稳定性、高选择性的催化剂. 虽然贵金属催化剂的活性及稳定性较高, 但其价格昂贵、抗中毒性能差等缺点限制了其广泛应用. 因此, 具有价廉易得、抗中毒性能优良、不饱和d轨道等优点的过渡金属催化剂逐渐成为研究热点. 常见的过渡金属催化剂有Cu、Mn、Fe、Co、Ti等, 其中, 锰氧化物(MnOx)既具有非化学计量组成、多晶型、储氧/释氧能力强、低成本和环境相容性等常规特点, 还具有不同的[MnO6]八面体单元三维堆积[14-15]及多晶型组合[16]、化学缺陷的可调性、优异的低温催化活性等重要特性, 从而在催化氧化VOCs领域具有极大的潜力.
氧空位(Oxygen Vacancy, OVs)是指金属氧化物或其他含氧化合物中, 晶格中的氧原子(氧离子)脱离, 形成的空位. 根据不同的空间位置可分为表面、次表面及体相氧空位. 研究发现, OVs能有效改善材料的物化特性, 包括对电子结构[17]、几何结构、吸光能力[18-19]、磁性[20-21]等特性的影响, 因而在电子、能源、传感器等领域倍受关注. 作为一种结构缺陷, OVs往往呈亚稳定状态, 其周围原子会通过重排来维持界面能最低的稳定态, 进而出现新的活性位点[22], 并有可能在OVs附近出现豫驰现象[23-24].通常, 锰基催化剂催化氧化VOCs遵循Marse-van Krevelen(MVK)机理, 而OVs的涌现及湮灭在整个机理中起着非常重要的作用. 其中, 带正电的OVs会引入不成对电子, 导致表面电子密度增强, 起到类似于贵金属的作用[25], 进而加快对活性氧物种的补充. 近年来, 富含OVs的锰氧化物在催化氧化VOCs领域引发了广泛的研究, 然而, 有关氧空位的构建及表征方法, 特别是锰氧化物中OVs在催化氧化BTEX中具体作用的综述论文较少. 因此, 我们总结了OVs常见的构建方法及表征技术, 阐释了锰氧化物中OVs在催化降解BTEX中的几种作用, 并对富氧空位锰氧化物在该领域的未来机遇与挑战提出了展望.
1 氧空位的构建随着科学技术的不断发展, 有关OVs的构建方法日新月异. 目前, 催化剂中OVs的构建思路主要有掺杂异原子和去除氧原子(离子)两种.
1.1 还原法还原法是脱除氧原子(离子)的典型方法. 气相还原主要是利用还原性气体(H2、CO、NH3等)处理材料, 通过控制处理浓度[26]、处理温度[27]等参数实现不同浓度OVs的构建. 此外, 通过外加物质, 使其热解营造还原气氛[19], 也能起到类似的效果. Xu等[28]通过调节尿素和催化剂的质量比, 并在高温条件下煅烧, 既实现了铜锰复合氧化物中不同浓度OVs的构建, 又避免了尿素及其热解中间物对材料的影响. 同样的, 固相还原法是添加强还原剂(锂单质[29]、硼氢化钠[30]等)夺取催化剂中的氧, 在室温下实现金属氧化物(MO)中不同浓度OVs的构建, 其反应机理如下:
| $ {\rm{M}}{{\rm{O}}_y} + 2x{\rm{Li}} \to {\rm{M}}{{\rm{O}}_{y - x}} + x{\rm{L}}{{\rm{i}}_2}{\rm{O}} $ | (1) |
| $ {\rm{MO}} + \frac{x}{8}{\rm{NaB}}{{\rm{H}}_4} + \frac{x}{4}{{\rm{H}}_2}{\rm{O}} = {\rm{M}}{{\rm{O}}_{1 - x}}{({\rm{OH}})_x} + \frac{x}{8}{\rm{NaB}}{{\rm{O}}_2} $ | (2) |
另外, 添加弱还原剂(碳粉[31]、硫粉[32]、抗坏血酸[33]等)并在高温条件煅烧也能夺取氧从而构建OVs. 溶剂热及水热法是液相还原法中代表性的方法, 其中, 醇类既是一种分散剂也是一种还原剂. Wu等[34]以乙二醇为还原剂, 通过溶剂-热还原方法制备了富氧空位MnO2纳米片(MnO2-PS), 其表现出最强的电子顺磁共振信号, 表明其具有最多的氧空位(图 1). 与气相还原法类似, 调节反应时间[35]、还原剂种类[36]等参数同样能实现不同浓度OVs的构建.

|
图 1 MnO2-PS、MnO2-R和MnO2-B催化剂g因子的低温EPR谱[34] Fig.1 Low-temperature EPR spectra of g factor for theMnO2-PS, MnO2-R and MnO2-B catalysts[34] |
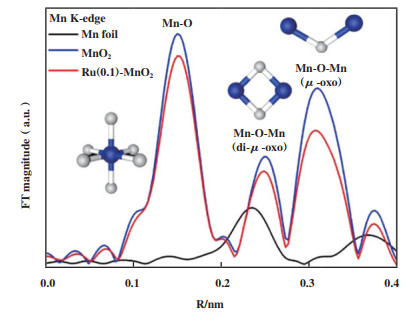
大量的研究发现, 异金属离子掺杂不仅能增强金属间的相互作用, 促进电子转移, 还能造成晶格畸变[37-38], 打破材料的静电平衡, 促使OVs的产生[39]. Jia等[40]利用水热合成法将Fe掺入到MnO2晶格当中, 进而引入丰富的氧空位. 同样地, Gu等[41]将Ru掺杂到β-MnO2隧道结构中, 通过扩展X射线吸收精细结构发现锰价态的降低, 证明其氧配位数减少(图 2), 并结合EPR证实了Ru的引入能促进β-MnO2中氧空位的产生. 虽然有研究发现OVs浓度与掺杂量呈正相关[42], 但Ren等[43]发现OVs浓度会随Ce3+掺杂量呈火山形趋势. 因此, 掺杂量和氧空位之间的相关性有待进一步证实, 但值得注意的是, OVs确实与催化性能间存在密不可分的联系[28, 44].
|
图 2 Ru(0.1)-MnO2、Mn箔和MnO2的傅里叶变换EXAFS光谱[41] Fig.2 Fourier-transform EXAFS spectra of Ru(0.1)-MnO2, Mn foil and MnO2[41] |
近年来, 科研人员通过调节煅烧气氛中氧分压来引入氧空位. Yang等[45]在空气、N2和Ar气氛条件下煅烧后的锰氧化物具有不同含量的OVs. Lu等[46]通过调节不同百分比的氧分压实现了Mn3O4中的OVs浓度的调控. Fang等[47]对预煅烧样品进行二次真空煅烧, 成功地在α-MnO2中引入了氧空位. 整个过程可以用标准的Kröger-Vink符号来描述, OVs的产生及其浓度的定义方程如下[48]:
| $ {{\rm{O}}_{\rm{L}}} \leftrightarrow {\rm{OVs}} + 0.5{{\rm{O}}_2} + 2{\rm{e}} $ | (3) |
| $ {{\rm{C}}_{{{\rm{V}}_0}}} = {{\rm{K}}^*}{{\rm{n}}^{ - 2}}*{\rm{p}}_{{{\rm{O}}_2}}^{ - 0.5} $ | (4) |
式中OL、OVs、CVO、pO2、K分别表示晶格氧、氧空位、OVs浓度、氧分压和式(3)的平衡常数. 由式(4)可知, VO的浓度与O2压力呈负相关, 说明缺氧气氛下有利于VO的形成. 然而, 不同类型的惰性气氛却呈现不同的效果, 其中机理还没有得到明确的解释. 除了控制气相中的氧分压外, 营造低分压的介质也能促进OVs的形成. Wang等[49]以熔融盐法合成富氧空位的层状MnO2, 这主要是因为熔融态能提供较低的氧分压[50], 进而产生大量氧空位. 几种构建方法(还原法、金属离子掺杂和氧分压调节)及其控制参数和优缺点, 如表 1所示. 此外, 针对现有的OVs表征技术, 我们列举了扫描隧道显微镜、X射线光电子能谱、拉曼、电子顺磁共振、电子自旋共振、电子能量损失光谱、X射线吸收精细结构、X射线吸收近边光谱、正电子湮灭寿命谱、光致发光光谱的表征原理及优缺点, 如表 2所示.
| 表 1 氧空位构建方法及对比分析表 Table 1 Oxygen vacancy construction method and comparative analysis table |
| 表 2 氧空位的表征技术对比分析表 Table 2 Comparative analysis table of characterization techniques of OVs |
作为一种重要的过渡金属, 锰氧化物不仅能通过上述方法构建OVs, 还能通过合成不同晶相、不同混合晶相等方式实现各价态间的转变及OVs的引入, 进而对热催化氧化BTEX表现出积极的促进作用. 随着研究的深入, 研究人员发现锰氧化物中OVs可同时起到多种作用, 具体如下所示.
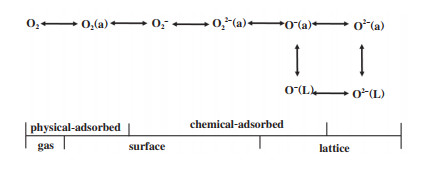
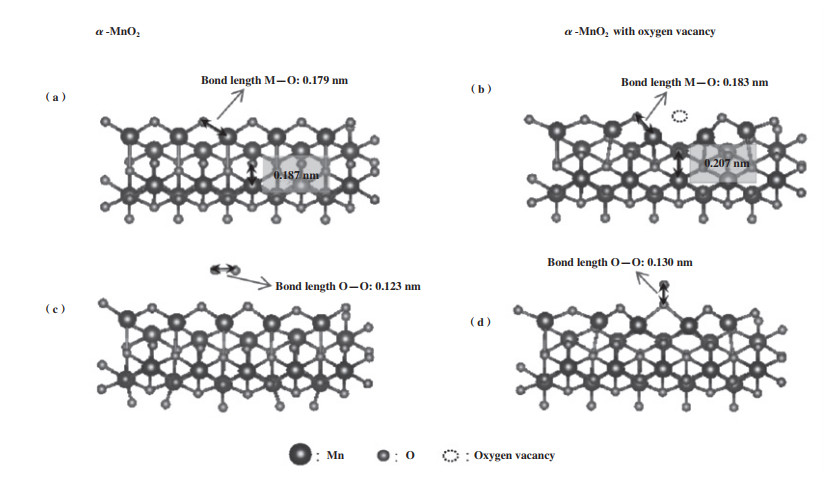
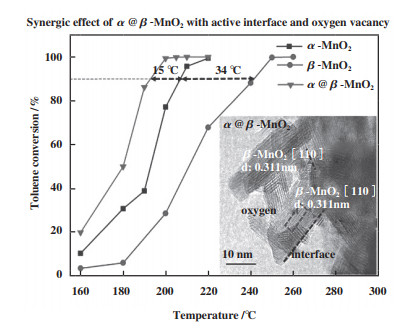
2.1 氧空位的吸附及活化作用 2.1.1 氧空位对O2的吸附及活化作用在实际的工业应用中, 催化剂的稳定性是非常重要的一项指标, 但催化剂本身的氧物种十分有限, 若没有O2的补充, 很难实现BTEX长时间的催化降解. 因此, O2的吸附及活化显得尤为重要. 相关研究发现, OVs不仅能提供O2的捕获位点, 还能促进电子转移[58]使O2活化成各类活性氧物种(Reactive oxygen species, ROS)[59-62]. 其中, 不同程度的电子转移能形成不同的氧物种[63-64], 如图 3所示, 从O2到O2-再到O2-过程伴随着反应活性的不断增强. 因此, 催化剂的ROS数量与OVs浓度存在一定的关系[65], 即通过表面氧物种数量来间接判断氧空位的数量[29]. 同时, Liu等[33]和Chen等[66]通过DFT理论计算证明了OVs能降低O2的吸附能, 弱化O-O键, 进而促进其吸附及活化, 如图 4(c)、(d)所示. 除此以外, Ma等[67]利用同位素氧交换和氧气程序升温脱附(O2-TPD)联合实验证明OVs不仅能促进O2形成ROS, 还能通过氧异交换反应(18O2(g)+16O(s)→16O18O(g)+18O(s))与晶格氧发生氧交换, 进而促进晶格氧物种的补充(图 3). 同样地, Dong等[68]通过设计原位的程序升温实验证明了O2可以优先补充体相晶格氧, 说明OVs既能活化O2为吸附氧物种, 又能进一步活化为晶格氧物种. Huang等[69]合成了具有分级α@β晶相的纳米MnO2催化剂, 界面处丰富的OVs能有效促进O2的吸附及活化, 0.05%甲苯可在205 ℃实现完全催化氧化(图 5).
|
图 3 氧气在锰氧化物表面的不同吸附形态[60] Fig.3 Different adsorption forms of oxygen on the surface of manganese oxide[60] |
|
图 4 α-MnO2中不含/含氧空位的晶格结构计算图[66] Fig.4 Calculation of lattice structure of α-MnO2 without/with oxygen vacancies[66] |
|
图 5 3个样品甲苯氧化活性随温度变化的分布图[69] Fig.5 Activity profiles of the three samples for toluene oxidation as a function of temperature[69] |
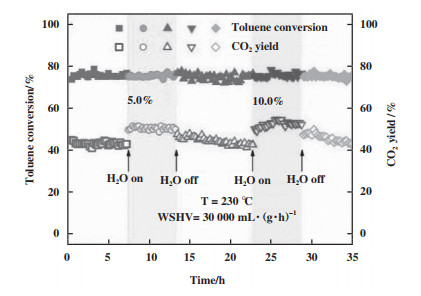
有研究发现, H2O也会被OVs活化, 形成羟基(·OH)等具有强氧化性的物种[30, 70-71]. Schaub等[52]利用STM证实了在暴露于水前后, OVs和·OH存在明显关系, 说明水被OVs解离吸附, 并通过质子转移到相邻的桥接氧原子, 产生两个桥接羟基. Zhang等[72]通过H2O-TPD发现H2O能吸附到OVs上形成HOH活性位点, 并提出活性氧能和结合水形成·OH, 进而促进氧化反应, 这说明H2O可以和OVs周围氧物种作用, 形成羟基. 此外, 吸附态的氧能通过氢转移反应(O2* + H2O* → OOH* + OH*和OOH*→O* + OH*)[73]形成·OH, 进而促进O2的活化. 为了探究水引入后对催化活性的影响, 他们结合原位漫反射傅里叶变换红外光谱(in-suit DRIFTS)、C7H8-TPD、甲苯程序升温表面反应(C7H8-TPSR)、DFT理论计算证明了水有利于O2的活化, 推动吸附氧向晶格氧的转化, 进而促进甲苯的矿化(图 6). 虽然OVs活化H2O能在一定程度上提升催化剂的抗水性能, 甚至是促进催化反应, 但过量的H2O会与O2和苯系物之间存在竞争吸附[73], 进而引起催化剂失活.
|
图 6 230 ℃水蒸气对3Mn2Ce催化性能的影响[72] Fig.6 The effect of water vapor onto catalytic performance of 3Mn2Ce at 230 ℃[72] |
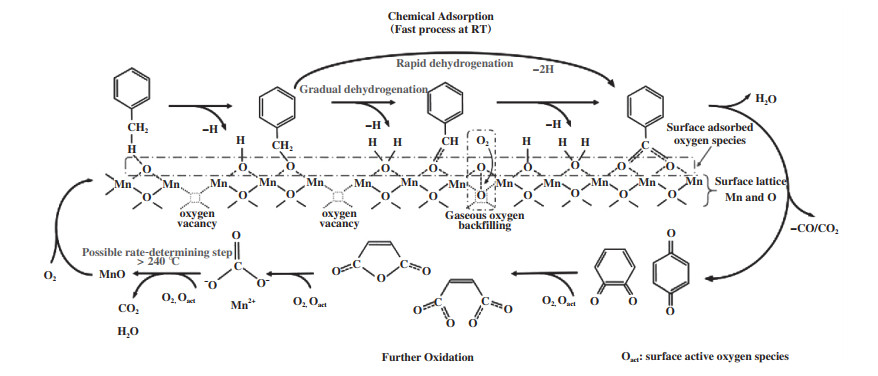
除了以上提到的两种作用外, OVs的存在还有助于BTEX的吸附[68]、活化, 其中最合理的解释就是OVs促进ROS的形成, 并与苯系物相互作用来提升其吸附及活化性能[74]. Yang等[14]利用in-suit DRIFTS发现δ-MnO2上氧空位能吸附大量的ROS, 这能促使甲苯上的甲基快速脱氢, 进而促进甲苯的吸附(图 7). 同样地, Liu等[75]得出相同的结论, 在吸附及活化苯系物后, 通过不断地活化O2产生更多的活性氧物种, 促进更深度的反应, 减少中间副产物的生成, 甚至改变苯系物的降解路径[76], 最终有利于污染物的完全矿化.
|
图 7 甲苯在δ-MnO2上吸附和氧化的可能机理[14] Fig.7 The probable mechanisms of toluene adsorption and oxidation over δ-MnO2[14] |
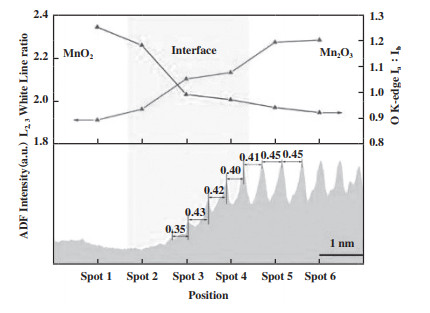
针对高分子量的BTEX, 仅利用锰氧化物的ROS能有效促进污染物的吸附及活化, 很难实现其完全矿化, 这主要是因为苯环具有相对稳定的结构, 其裂解需要严苛的条件. 同时, 从苯环裂解到实现完全催化氧化的过程中伴随着复杂的中间产物, 也因此成为了BTEX催化氧化过程中非常重要的速控步骤. 由于锰氧化物在催化氧化物VOCs的过程中遵循MVK机理, 因此充分利用晶格氧物种对BTEX的深度氧化至关重要[77-79]. MVK机理就是催化剂表面吸附的VOCs与晶格氧反应生成CO2和H2O, 同时催化剂被还原, 产生OVs, 随后气相氧补充OVs, 催化剂被氧化, 进而实现OVs的涌现-湮灭循环. 相关研究发现, OVs对晶格氧具有活化作用, 使其易于迁移至催化剂表面参与催化反应[80], 这主要是因为与OVs相邻的晶格氧可以被泵送以补充空位, 从而提高晶格氧物种的迁移率[81]. Ni等[82]证实在OMS-2中掺杂Sr2+会导致OVs的形成, 促进晶格氧的活化, 进而提升对苯的催化性能, 随后利用同位素氧交换实验证实了整个反应遵循MVK机理. 随着相关研究的不断推进, 科研人员发现不同位置的OVs在催化氧化BTEX的过程中扮演着不同的角色. Su等[83]发现CeO2中表面OVs倾向于促进O2的活化和吸附氧的形成, 而体相OVs倾向于增强晶格氧物种的活性和迁移, 并在高温条件下参与污染物的进一步分解, 这一点也被其它[77]研究证实, 即锰氧化物中晶格氧物种需要在高温条件下参与反应. Yang等[16]通过构建MnO2和Mn2O3的异质界面, 结合高角度环形暗场扫描透射电子显微镜(HAADF-STEM)和EELS技术对界面附近的锰原子柱间距、锰配位(Ia/Ib)和价态(IL3/IL2)进行分析(图 8), 发现界面处产生丰富的OVs, 进而提供大量吸附氧物种和冗余晶格氧. 结合in-suit DRIFTS分析反应机理, OVs吸附的活性氧物种促进甲苯的甲基脱氢和去甲基化, 形成重要的中间产物, 冗余配位晶格氧有助于增强苯环的断裂能力.
|
图 8 O K边前峰的Ia∶Ib和Mn L2, 3白线比与相应的ADF信号强度作为T-0.5上不同位置的函数及锰原子柱间距[16] Fig.8 Ia∶Ib of O K-edge prepeaks and Mn L2, 3 white line ratio with corresponding ADF signal intensity as functions of different positions on T-0.5 and manganese column spacing[16] |
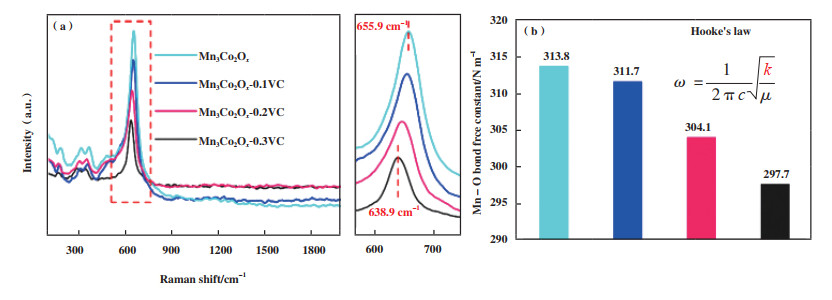
由于氧元素的缺失, 催化剂整体电荷平衡被打破, 为了保持电荷平衡, 金属氧化物的价态往往会发生变化. 对于锰氧化物而言, 在光电子发射过程中, 三维电子与3s电子之间的平行自旋耦合导致Mn 3s光谱分裂, 通过测试Mn 3s多重态分裂能(ΔE), 最后根据公式(5)计算Mn的平均氧化态(Average Oxidation State, AOS). 通常情况下, 较高OVs浓度往往伴随着低价态锰物种占比的增加, 即更低的AOS, 这也间接成为氧空位构建成功的判断依据. Zhu等[84]通过向α-MnO2纳米线隧道中引入高浓度的K+, 实现了晶胞的扩张、电荷平衡的破坏, 进而导致AOS的降低及OVs的出现. 各价态锰物种的形成促进材料表面的电子转移, 提升热催化氧化性能[85-86], 同时, 更低的AOS会通过Jahn-Teller效应拉长Mn-O键, 弱化其强度[33], 如图 9所示. 根据Raman的测试结果, 将638.9~655.9 cm-1范围内的拉曼位移代入胡克定律计算锰氧键合力常数, 并进一步计算平均Mn-O键长度[39], 公式如下:
| $ {\rm{AOS}} = 8.956 - 1.126*\Delta {\rm{E}} $ | (5) |
| $ \omega = \frac{1}{{2\pi c\sqrt {\frac{k}{\mu }} }} $ | (6) |
| $ k = \frac{{17}}{{{r^3}}} $ | (7) |
|
图 9 不同催化剂的拉曼光谱及基于胡克定律计算的Mn-O键力常数[33] Fig.9 Raman spectra of different catalysts and the calculated Mn-O bond force constant based on Hooke's law[33] |
其中ω、c、μ、k、r分别代表拉曼位移、光速、Mn-O键的有效质量、Mn-O键常数和平均Mn-O键长度. 更弱的Mn-O键致使氧离子更容易解离和活化(图 9(a), (b)), 最终导致更多的活性晶格氧参与反应[66].
综上所述, 锰氧化物种的OVs在催化氧化BTEX中起到不同的积极作用. 然而, OVs的作用可能远不止于此. 有研究提出OVs是活化的酸位点, 原子电负性的不平衡导致酸性增强[87], 进而有利于C-H键的断裂[88], 但关于锰氧化物中OVs对酸性的调节, 进而影响BTEX催化氧化作用的机理还有待详细研究.
3 总结与展望近年来, OVs被发现在催化氧化领域起到非常重要的作用, 我们虽综述了常见的OVs构建方法, 包括还原法、异金属离子掺杂、调节氧分压等, 但OVs的具体的形成过程和部分形成机理尚未探明, 仍需借助更有效的表征技术来探明, 同时, 研发更简单、更可控的构建方法以及如何在众多方法中挑选出符合实际条件的构建方法仍需进一步探究.
锰氧化物中OVs在热催化氧化BTEX中的具体作用已有许多研究, 包括OVs对O2、H2O、BTEX的吸附及活化, 对晶格氧的活化及迁移, 对催化剂价态结构的影响. 然而, 锰氧化物中OVs的研究仍存在诸多机遇和挑战, 具体包括: (1)提升催化剂的抗水性能是推进其工业应用的重要一步, 如何避免水蒸气对OVs的负面影响将会成为未来研究的热点之一; (2)相关研究发现吸附氧物种也能促进BTEX的吸附, 而吸附氧物种并非完全由OVs产生, 因此, 不同吸附氧物种的来源及其在吸附和活化BTEX上起到的作用是否存在异同也势必存在争议; (3)综上所述, 锰氧化物中OVs具有多种作用, 但有关众多作用之间相互关系的研究较少. 此外, 有研究发现OVs团簇的密度和大小对催化性能有直接影响, 但有关锰氧化物在此方面的研究较少; (4)关于锰氧化物中OVs对催化剂酸性及其它特性的作用还缺乏相应的研究; (5)BTEX种类繁多, 实际工业废气也由多种VOCs组成, 而锰氧化物在协同去除多组分苯系物废气方面的研究鲜有报道, 这些都将成为未来的研究方向.
| [1] |
Yi H H, Huang Y H, Tang X L, et al. Characteristics of non-methane hydrocarbons and benzene series emission from commonly cooking oil fumes[J]. Atmos Environ, 2019, 200(1): 208–220.
|
| [2] |
Yang Ting(杨婷), Li Dan-Dan(李丹丹), Shan Xuan-Long(单玄龙), et al. Pollution characterization, source apporti-onment and health risk assessment of benzene homolo-gues in the ambient air of a typical urban area in Beijing, China(北京市典型城区环境空气中苯系物的污染特征、来源分析与健康风险评价)[J]. Ecotoxicology(生态毒理学报), 2017, 12(5): 79–97.
|
| [3] |
Liu A, Hong N, Zhu P F, et al. Understanding benzene series(BTEX) pollutant load characteristics in the urban environment[J]. Sci Total Environ, 2018, 619/620(1): 938–945.
|
| [4] |
Armenta-Resendiz M, Rios-Leal E, Rivera-Garcia M T, et al. Structure-activity study of acute neurobehavioral effects of cyclohexane, benzene, m-xylene and toluene in rats[J]. Toxicol Appl Pharmacol, 2019, 376(1): 38–45.
|
| [5] |
Abdel Maksoud H A, Elharrif M G, Mahfouz M K, et al. Biochemical study on occupational inhalation of benzene vapours in petrol station[J]. Respir Med Case Rep, 2019, 27: 100836.
|
| [6] |
Mousavi N, Tafvizi F, Mansoori Y. Genetic polymorphisms of base excision repair gene XRCC1 and susceptibility to benzene among employees of chemical industries[J]. Gene Rep, 2021, 23: 101081.
DOI:10.1016/j.genrep.2021.101081 |
| [7] |
Shallis R M, Weiss J J, Deziel N C, et al. A clandestine culprit with critical consequences: Benzene and acute myeloid leukemia[J]. Blood Rev, 2021, 47: 100736.
DOI:10.1016/j.blre.2020.100736 |
| [8] |
Yao S, Wang Q H, Zhang J M, et al. Ambient volatile organic compounds in a heavy industrial city: Concentr-ation, ozone formation potential, sources and health risk assessment[J]. Atmos Pollut Res, 2021, 12(5): 101053.
DOI:10.1016/j.apr.2021.101053 |
| [9] |
Xuan L C, Ma Y N, Xing Y F, et al. Source, temporal variation and health risk of volatile organic compounds (VOCs) from urban traffic in harbin, China[J]. Environ Pollut, 2021, 270(1): 116074.
|
| [10] |
AL-Naiema I M, Offenberg J H, Madler C J, et al. Secon-dary organic aerosols from aromatic hydrocarbons and their contribution to fine particulate matter in Atlanta, Georgia[J]. Atmospheric Environ, 2020, 223(15): 117227.
|
| [11] |
a. Liu R, Tian M Z, Shang W, et al. Normal temperature catalytic degradation of toluene over Pt/TiO2[J]. Environ Technol, 2020, 43(13): 2047-2058. b. Liu Yu-feng(刘玉凤),, Zhou Ying(周瑛),, Lu Mei(卢梅), et al. Preparation of noble metal single-atom catalystand its applications in catalytic oxidation reaction of CO and VOCs(贵金属单原子催化剂的制备及其在CO、VOCs完全氧化反应中的应用)[J]. J Mol Catal (China)(分子催化), 2022, 36(1): 81-97. c. Zhang Ying(张迎), Zhu Wen-jie(朱文杰), Fu Te(富特), et al. Research progress of Ce-based catalysts in catalytic combustion control of volatile organic compounds(铈基催化剂在挥发性有机物催化燃烧治理中的研究进展)[J]. J Mol Catal (China)(分子催化), 2022, 36(1): 58-70. |
| [12] |
Holzer F, Kopinke F D, Roland U. Non-thermal plasma treatment for the elimination of odorous compounds from exhaust air from cooking processes[J]. Chem Eng J, 2018, 334(15): 1988–1995.
|
| [13] |
Padhi S K, Gokhale S. Treatment of gaseous volatile organiccompounds using a rotating biological filter[J]. BioresourTechnol, 2017, 244: 270–280.
DOI:10.1016/j.biortech.2017.07.112 |
| [14] |
Yang W H, Su Z A, Xu Z H, et al. Comparative study of α-, β-, γ-and δ-MnO2 on toluene oxidation: Oxygen vacan-cies and reaction intermediates[J]. Appl Catal B, 2020, 260: 118150.
DOI:10.1016/j.apcatb.2019.118150 |
| [15] |
Yang R J, Guo Z J, Cai L X, et al. Investigation into thephase-activity relationship of MnO2 nanomaterials toward ozone-assisted catalytic oxidation of toluene[J]. Small, 2021, 17(50): 2103052.
DOI:10.1002/smll.202103052 |
| [16] |
Yang W H, Peng Y, Wang Y, et al. Controllable redox-induced in-situ growth of MnO2 over Mn2O3 for toluene oxidation: Active heterostructure interfaces[J]. Appl Catal B, 2020, 278(5): 119279.
|
| [17] |
Han F F, Xu J, Zhou J, et al. Oxygen vacancy-engineered Fe2O3 nanoarrays as free-standing electrodes for flexible asymmetric supercapacitors[J]. Nanoscale, 2019, 11(26): 12477–12483.
DOI:10.1039/C9NR04023D |
| [18] |
Lahmer M A. Effect of oxygen vacancies on the electronic structure and dielectric properties of SrAl2O4:A first-principles study[J]. J Phys Chem Solids, 2022, 160: 110297.
DOI:10.1016/j.jpcs.2021.110297 |
| [19] |
Li Y J, Liu T, Cheng Z Y, et al. Facile synthesis of highcrystallinity and oxygen vacancies rich bismuth oxybro-mide up conversion nanosheets by air-annealing for UV-Vis-NIR broad spectrum driven Bisphenol A degradation[J]. Chem Eng J, 2020, 421: 127868.
|
| [20] |
Wang Z H, Yuan Q, Zhang Y F, et al. Oxygen vacancy-induced short-range ordering as a sole mechanism for enhanced magnetism of spinel ZnFe2O4 prepared via aonestep treatment[J]. Ceram Int, 2021, 47(13): 17877–17882.
DOI:10.1016/j.ceramint.2021.03.100 |
| [21] |
Shi L R, Yan X, Ju L W, et al. The effect of oxygen vacan-cy on magnetism of geometrically frustrated triangular lattice CuFeO2:Ab initio study[J]. J Magn Magn Mater, 2019, 486(15): 165295.
|
| [22] |
An X Q, Tang Q W, Lan H C, et al. Polyoxometalates/TiO2 Fenton-like photocatalysts with rearranged oxygen vacancies for enhanced synergetic degradation[J]. Appl Catal B, 2019, 244(5): 407–413.
|
| [23] |
Hirschle C, Schreuer J. High-temperature ultrasound attenuation in langasite and langatate[J]. IEEE Trans Ultrason Ferroelectr Freq Control, 2018, 65(7): 1250–1257.
DOI:10.1109/TUFFC.2018.2836434 |
| [24] |
Mativenga M, Haque F, Billah M M, et al. Origin of light instability in amorphous IGZO thin-film transistors and its suppression[J]. Sci Rep, 2021, 11(1): 14618.
DOI:10.1038/s41598-021-94078-8 |
| [25] |
a. Wang Y, Wu J, Wang G, et al. Oxygen vacancy engine-ering in Fe doped akhtenskite-type MnO2 for low-temper-ature toluene oxidation[J]. Appl Catal B, 2021, 285(15): 119873. b. Bi Xiu ru(毕秀茹), Meng Xu(孟旭), Zhao Pei qing(赵培庆). Application of manganese oxide octahedral molec-ular sieves(OMS 2) in clean synthesis of organic compounds(氧化锰八面体分子筛(OMS 2)在有机分子清洁合成中的催化氧化应用)[J]. J Mol Catal (China)(分子催化), 2020, 34(5): 462-474. c. Yang Yang (杨洋), Hu Zhun(胡准), Mi Rong li(米容立), et al. Effect of Mn loading on catalytic performance of nMnOx/TiO2 in NH3 SCR reaction(Mn负载量对nMnOx/TiO2催化剂NH3 SCR催化性能的影响)[J]. J Mol Catal (China)(分子催化), 2020, 34(4): 313-325. |
| [26] |
Lan Y P, Xia X W, Li J Q, et al. Insight into the contribu-tions of surface oxygen vacancies on the promoted photocatalytic property of nanoceria[J]. Nanomaterials-Basel, 2021, 11(5): 1168.
DOI:10.3390/nano11051168 |
| [27] |
Dai J, Shao N N, Zhang S W, et al. Enhanced piezoca-talytic activity of Sr0.5Ba0.5Nb2O6 nanostructures by engineering surface oxygen vacancies and self-generated heterojunctions[J]. ACS Appl Mater Interfaces, 2021, 13(6): 7259–7267.
DOI:10.1021/acsami.0c21202 |
| [28] |
Xu Y, Qu Z P, Ren Y W, et al. Enhancement of toluene oxida-tion performance over Cu-Mn composite oxides by regulating oxygen vacancy[J]. Appl Surf Sci, 2021, 560: 149983.
DOI:10.1016/j.apsusc.2021.149983 |
| [29] |
Ou G, Xu Y S, Wen B, et al. Tuning defects in oxides atroom temperature by lithium reduction[J]. Nat Comm-un, 2018, 9(1): 1302.
DOI:10.1038/s41467-018-03765-0 |
| [30] |
He M, Ji J, Liu B Y, et al. Reduced TiO2 with tunable oxygenvacancies for catalytic oxidation of formaldehyde at room temperature[J]. Appl Surf Sci, 2019, 473: 934–942.
DOI:10.1016/j.apsusc.2018.12.212 |
| [31] |
Zhou Y, Yan M K, Hou J X, et al. Introduction of oxygen vacancies into hematite in local reducing atmosphere for solar water oxidation[J]. Sol Energy, 2019, 179: 99–105.
DOI:10.1016/j.solener.2018.12.059 |
| [32] |
Wang F, Mao L M, Qi X, et al. Sulfur-induced abundant oxygen vacancies in hollow silica microsphere toward super anode[J]. Chem Eng J, 2021, 418: 129397.
DOI:10.1016/j.cej.2021.129397 |
| [33] |
Liu H, Jia W L, Yu X, et al. Vitamin c-assisted synthe-sized Mn-Co oxides with improved oxygen vacancy concentration: Boosting lattice oxygen activity for the air-oxidation of 5-(hydroxymethyl)furfural[J]. ACS Catal, 2021, 11(13): 7828–7844.
DOI:10.1021/acscatal.0c04503 |
| [34] |
Wu S P, Liu H M, Huang Z, et al. O-vacancy-rich porous MnO2 nanosheets as highly efficient catalysts for propane catalytic oxidation[J]. Appl Catal B, 2022, 312: 121387.
DOI:10.1016/j.apcatb.2022.121387 |
| [35] |
Zou P, Li Z G, Jia P Q, et al. Enhanced photocatalytic activity of bismuth oxychloride by in-situ introducing oxygen vacancy[J]. Coll Surf A Physicochem Eng Asp, 2021, 623: 126705.
DOI:10.1016/j.colsurfa.2021.126705 |
| [36] |
Dong C, Wang H, Ren Y W, et al. Layer MnO2 with oxygenvacancy for improved toluene oxidation activity[J]. Surf Interf, 2021, 22: 100897.
DOI:10.1016/j.surfin.2020.100897 |
| [37] |
Anbuselan D, Nilavazhagan S, Santhanam A, et al. The effect of iron doping on the structural, optical, surface morphological and temperature-dependent magnetic properties of ZnO nanoparticles[J]. J Phys Condens, 2021, 33(9): 094001.
DOI:10.1088/1361-648X/abcd80 |
| [38] |
Peng K, Hou Y Q, Zhang Y Z, et al. Engineering oxygen vacancies in metal-doped MnO2 nanospheres for boosting the low-temperature toluene oxidation[J]. Fuel, 2022, 314: 123123.
DOI:10.1016/j.fuel.2021.123123 |
| [39] |
Ma M, Zhu Q, Jiang Z Y, et al. Achieving toluene efficient mineralization over K/α-MnO2 via oxygen vacancy modu-lation[J]. J Colloid Interface Sci, 2021, 598(15): 238–249.
|
| [40] |
Jia J B, Yang W J, Zhang P Y, et al. Facile synthesis ofFe-modified manganese oxide with high content of oxygen vacancies for efficient airborne ozone destruction[J]. Appl Catal A, 2017, 546: 79–86.
DOI:10.1016/j.apcata.2017.08.013 |
| [41] |
Gu Y D, Min Y X, Li L, et al. Crystal splintering of β-MnO2 induced by interstitial Ru doping toward reversible oxygen conversion[J]. Chem Mater, 2021, 33(11): 4135–4145.
DOI:10.1021/acs.chemmater.1c00828 |
| [42] |
Dong C, Qu Z P, Jiang X, et al. Tuning oxygen vacancy concentration of MnO2 through metal doping for improved toluene oxidation[J]. J Hazard Mater, 2020, 391: 122181.
DOI:10.1016/j.jhazmat.2020.122181 |
| [43] |
Ren X X, Gao Y G, Zheng L R, et al. Oxygen vacancy enha-ncing CO2 electrochemical reduction to CO on Cedoped ZnO catalysts[J]. Surf Interface Anal, 2021, 23: 100923.
DOI:10.1016/j.surfin.2020.100923 |
| [44] |
Bo Y N, Wang H Y, Lin Y X, et al. Altering hydroge-nation pathways in photocatalytic nitrogen fixation by tuning local electronic structure of oxygen vacancy with dopant[J]. Angew Chem Int Ed, 2021, 60(29): 16085–16092.
DOI:10.1002/anie.202104001 |
| [45] |
Yang R C, Ren C, Teng X G, et al. Study of the surface oxygen vacancies evolvement on the single and bi-comp-onents manganese oxide precursors and their catalytic performance[J]. Catal Lett, 2017, 147(3): 727–737.
DOI:10.1007/s10562-017-1973-0 |
| [46] |
Lu W H, Chen J, Kong L S, et al. Oxygen vacancies modulation Mn3O4 nanozyme with enhanced oxidase-mimicking performance for l-cysteine detection[J]. Sensor Actuat B-Chem, 2021, 333: 129560.
DOI:10.1016/j.snb.2021.129560 |
| [47] |
Fang R M, Huang J, Huang X Y, et al. Reheat treatment under vacuum induces pre-calcined alpha-MnO2 with oxygen vacancy as efficient catalysts for toluene oxidation[J]. Chemosphere, 2022, 289: 133081.
DOI:10.1016/j.chemosphere.2021.133081 |
| [48] |
Wang L, Xie X, Dinh K N, et al. Synthesis, characteri-zations and utilization of oxygen-deficient metal oxides for lithium/sodium-ion batteries and supercapacitors[J]. Coord Chem Rev, 2019, 397: 138–167.
DOI:10.1016/j.ccr.2019.06.015 |
| [49] |
Wang Y, Liu K S, Wu J, et al. Unveiling the effects ofalkali metal ions intercalated in layered MnO2 for formal-dehyde catalytic oxidation[J]. ACS Catal, 2020, 10(17): 10021–10031.
DOI:10.1021/acscatal.0c02310 |
| [50] |
Li L Y, Liu Y C, Yu H B, et al. Hollow spherical 0.5Li2MnO3·0.5LiMn1/3Ni1/3Co1/3O2 prepared by facile molten salt method for enhanced long-cycle and rate capability of lithium-ion batteries[J]. J Alloys Compd, 2021, 855: 157376.
DOI:10.1016/j.jallcom.2020.157376 |
| [51] |
Yu Y, Liu S L, Ji J, et al. Amorphous MnO2 surviving calc-ination: an efficient catalyst for ozone decomposition[J]. Catal Sci Technol, 2019, 9(18): 5090–5099.
DOI:10.1039/C9CY01426H |
| [52] |
Schaub R, Thostrup P, Lopez N, et al. Oxygen vacancies as active sites for water dissociation on rutile TiO2(110)[J]. Phys Rev Lett, 2001, 87(26): 266104.
DOI:10.1103/PhysRevLett.87.266104 |
| [53] |
Lin X T, Li S J, He H, et al. Evolution of oxygen vacanc-ies in MnOx-CeO2 mixed oxides for soot oxidation[J]. Appl Catal B, 2018, 223: 91–102.
DOI:10.1016/j.apcatb.2017.06.071 |
| [54] |
Liu H J, Fu H, Liu Y C, et al. Synthesis, characterization and utilization of oxygen vacancy contained metal oxide semiconductors for energy and environmental catalysis[J]. Chemosphere, 2021, 272: 129534.
DOI:10.1016/j.chemosphere.2021.129534 |
| [55] |
Li G G, Li N, Sun Y G, et al. Efficient defect engineering in Co-Mn binary oxides for low-temperature propane oxidation[J]. Appl Catal B, 2021, 282: 119512.
DOI:10.1016/j.apcatb.2020.119512 |
| [56] |
Ye K H, Li K S, Lu Y R, et al. An overview of advanced methods for the characterization of oxygen vacancies in materials[J]. Trends Analyt Chem, 2019, 116: 102–108.
DOI:10.1016/j.trac.2019.05.002 |
| [57] |
Kernazhitsky L, Shymanovska V, Naumov V, et al. Room temperature photoluminescence of mixed titaniumman-ganese oxides[J]. J Lumin, 2017, 187: 521–527.
DOI:10.1016/j.jlumin.2017.03.015 |
| [58] |
Zheng Y F, Liu Q L, Shan C P, et al. Defective ultrafine MnOx nanoparticles confined within a carbon matrix for low-temperature oxidation of volatile organic compounds[J]. Environ Sci Technol, 2021, 55(8): 5403–5411.
DOI:10.1021/acs.est.0c08335 |
| [59] |
Xu H M, Yan N Q, Qu Z, et al. Gaseous heterogeneous catalytic reactions over Mn-based oxides for environm-ental applications: A critical review[J]. Environ Sci Technol, 2017, 51(16): 8879–8892.
DOI:10.1021/acs.est.6b06079 |
| [60] |
Zhang Y Z, Li Y F, Zeng Z Q, et al. Synergically engine-ering Cu+ and oxygen vacancies in CuMn2O4 catalsts for enhanced toluene oxidation performance[J]. Mol Catal, 2022, 517: 112043.
DOI:10.1016/j.mcat.2021.112043 |
| [61] |
Du Y K, Zou J W, Guo Y, et al. A novel viewpoint on thesurface adsorbed oxygen and the atom doping in the catalytic oxidation of toluene over low-Pt bimetal catalysts[J]. Appl Catal A, 2021, 609: 117913.
DOI:10.1016/j.apcata.2020.117913 |
| [62] |
Wang Y H, Zhang L Z, Zhang C S, et al. Promoting the generation of active oxygen over Ag-modified nanoflower-like α-MnO2 for soot oxidation: Experimental and DFT studies[J]. Ind Eng Chem Res, 2020, 59(22): 10407–10417.
DOI:10.1021/acs.iecr.0c01308 |
| [63] |
Yang W J, Zhu Y F, You F, et al. Insights into the surface-defect dependence of molecular oxygen activation over birnessite-type MnO2[J]. Appl Catal B, 2018, 233: 184–193.
DOI:10.1016/j.apcatb.2018.03.107 |
| [64] |
Yang J, Hu S Y, Fang Y R, et al. Oxygen vacancy promotedO2 activation over perovskite oxide for low-temperature CO oxidation[J]. ACS Catal, 2019, 9(11): 9751–9763.
DOI:10.1021/acscatal.9b02408 |
| [65] |
Wang G R, Zhang J, Zhou J Z, et al. Production of an effective catalyst with increased oxygen vacancies from manganese slag for selective catalytic reduction of nitric oxide[J]. J Environ Manage, 2019, 239: 90–95.
DOI:10.1016/j.jenvman.2019.03.056 |
| [66] |
Chen L Z, Liu Y J, Fang X, et al. Simple strategy for the construction of oxygen vacancies on alpha-MnO2 catalyst to improve toluene catalytic oxidation[J]. J Hazard Mater, 2021, 409: 125020.
DOI:10.1016/j.jhazmat.2020.125020 |
| [67] |
Ma X Y, Xiao M L, Yang X Q, et al. Boosting benzene combustion by engineering oxygen vacancy-mediated Ag/CeO2-Co3O4 catalyst via interfacial electron transfer[J]. J Coll Interf Sci, 2021, 594: 882–890.
DOI:10.1016/j.jcis.2021.03.076 |
| [68] |
Dong C, Qu Z P, Qin Y, et al. Revealing the highly catal-ytic performance of spinel CoMn2O4 for toluene oxidation: Involvement and replenishment of oxygen species using in situ designed-TP techniques[J]. ACS Catal, 2019, 9(8): 6698–6710.
DOI:10.1021/acscatal.9b01324 |
| [69] |
Huang N, Qu Z P, Dong C, et al. Superior performance of α@β-MnO2 for the toluene oxidation: Active interface and oxygen vacancy[J]. Appl Catal A, 2018, 560(25): 195–205.
|
| [70] |
He M, Cao Y Q, Ji J, et al. Superior catalytic performance of Pd-loaded oxygen-vacancy-rich TiO2 for formaldehyde oxidation at room temperature[J]. J Catal, 2021, 396: 122–135.
DOI:10.1016/j.jcat.2021.01.035 |
| [71] |
Ding J Y, Yang Y J, Liu J, et al. Mechanistic study on the role of oxygen vacancy for methylene chloride oxidation over La-Mn perovskite[J]. Appl Surf Sci, 2021, 559: 149979.
DOI:10.1016/j.apsusc.2021.149979 |
| [72] |
Zhang X D, Bi F K, Zhu Z Q, et al. The promoting effectof H2O on rod-like MnCeOx derived from MOFs for toluene oxidation: A combined experimental and theor-etical investigation[J]. Appl Catal B, 2021, 297: 149979.
|
| [73] |
Feng Y, Wang C C, Wang C, et al. Catalytic stability enha-ncement for pollutant removal via balancing lattice oxygen mobility and VOCs adsorption[J]. J Hazard Mater, 2022, 424: 127337.
DOI:10.1016/j.jhazmat.2021.127337 |
| [74] |
Zhang H Y, Sui S H, Zheng X M, et al. One-pot synthesis of atomically dispersed Pt on MnO2 for efficient catalytic decomposition of toluene at low temperatures[J]. Appl Catal B, 2019, 257: 117878.
DOI:10.1016/j.apcatb.2019.117878 |
| [75] |
Liu W, Xiang W J, Guan N N, et al. Enhanced catalytic performance for toluene purification over Co3O4/MnO2 catalyst through the construction of different Co3O4-MnO2 interface[J]. Sep Purif Technol, 2021, 278: 119590.
DOI:10.1016/j.seppur.2021.119590 |
| [76] |
Yang X Q, Yu X L, Jing M Z, et al. Defective MnxZr1-xO2solid solution for the catalytic oxidation of toluene: Insi-ghts into the oxygen vacancy contribution[J]. ACS Appl Mater Interfaces, 2019, 11(1): 730–739.
DOI:10.1021/acsami.8b17062 |
| [77] |
Qin Yuan(秦媛). Study of the performance of toluene catal-ytic oxidation and the cycle of oxygen species over Mn-based catalysts(锰基催化剂催化氧化甲苯性能及其氧物种循环过程的研究)[D]. Dalian(大连): Dalian University of Technology(大连理工大学), 2020.
|
| [78] |
Sun Y H, Fan J, Cheng H R, et al. Investigation into the roles of different oxygen species in toluene oxidation over manganese-supported platinum catalysts[J]. Mol Catal, 2021, 507: 111569.
DOI:10.1016/j.mcat.2021.111569 |
| [79] |
Chen J, Chen X, Yan D X, et al. A facile strategy of enha-ncing interaction between cerium and manganese oxides for catalytic removal of gaseous organic contaminants[J]. Appl Catal B, 2019, 250: 396–407.
DOI:10.1016/j.apcatb.2019.03.042 |
| [80] |
Chen F, Zhang P P, Xiao L W, et al. Structure-perform-ance correlations over Cu/ZnO interface for low-tempera-ture methanol synthesis from syngas containing CO2[J]. ACS Appl Mater Interfaces, 2021, 13(7): 8191–8205.
DOI:10.1021/acsami.0c18600 |
| [81] |
Zeng J, Xie H M, Liu Z, et al. Oxygen vacancy induced MnO2 catalysts for efficient toluene catalytic oxidation[J]. Catal Sci Technol, 2021, 11(20): 6708–6723.
DOI:10.1039/D1CY01274F |
| [82] |
Ni C L, Hou J T, Wang Z W, et al. Enhanced catalytic activity of OMS-2 for carcinogenic benzene elimination by tuning Sr2+ contents in the tunnels[J]. J Hazard Mater, 2020, 398: 122958.
DOI:10.1016/j.jhazmat.2020.122958 |
| [83] |
Su Z, Yang W H, Wang C Z, et al. Roles of oxygen vacan-cies in the bulk and surface of CeO2 for toluene catalytic combustion[J]. Environ Sci Technol, 2020, 54(19): 12684–12692.
DOI:10.1021/acs.est.0c03981 |
| [84] |
Zhu G X, Zhu J G, Li W L, et al. Tuning the K+ concentr-ation in the tunnels of alpha-MnO2 to increase the content of oxygen vacancy for ozone elimination[J]. Environ Sci Technol, 2018, 52(15): 8684–8692.
DOI:10.1021/acs.est.8b01594 |
| [85] |
Sihaib Z, Puleo F, Garcia-Vargas J M, et al. Manganese oxide-based catalysts for toluene oxidation[J]. Appl Catal B, 2017, 209: 689–700.
DOI:10.1016/j.apcatb.2017.03.042 |
| [86] |
Lee H J, Yang J H, You J H, et al. Sea-urchin-like mesoporous copper-manganese oxide catalysts: Influence of copper on benzene oxidation[J]. J Ind Eng Chem, 2020, 89: 156–165.
DOI:10.1016/j.jiec.2020.05.005 |
| [87] |
Kong J J, Xiang Z W, Li G Y, et al. Introduce oxygen vacancies into CeO2 catalyst for enhanced coke resistance during photothermocatalytic oxidation of typical VOCs[J]. Appl Catal B, 2020, 269: 118755.
DOI:10.1016/j.apcatb.2020.118755 |
| [88] |
Jian Y F, Tian M J, He C, et al. Efficient propane low-temperature destruction by Co3O4 crystal facets enginee-ring: Unveiling the decisive role of lattice and oxygen defects and surface acid-base pairs[J]. Appl Catal B, 2021, 283: 119657.
DOI:10.1016/j.apcatb.2020.119657 |
 2022, Vol. 36
2022, Vol. 36


