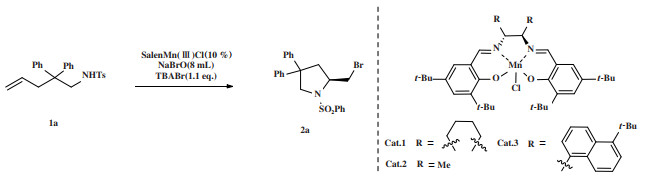
氮杂环化合物是一类重要的有机中间体, 是多种天然产物和人体必须物质的组成结构[1], 在生物医药领域有着广泛应用[2-3]. 卤代氮杂环由于卤素官能团的高反应活性, 可以通过多种反应转化成带有不同基团的物质, 是一类重要的化工产品[4-6]. 在氮杂环的合成中, 不饱和烃分子内胺化反应合成含氮杂环以其原子经济性和高反应活性而倍受重视[7]. 因此, 探究分子内胺化反应合成卤代氮杂环的新方法具有十分重要的意义.
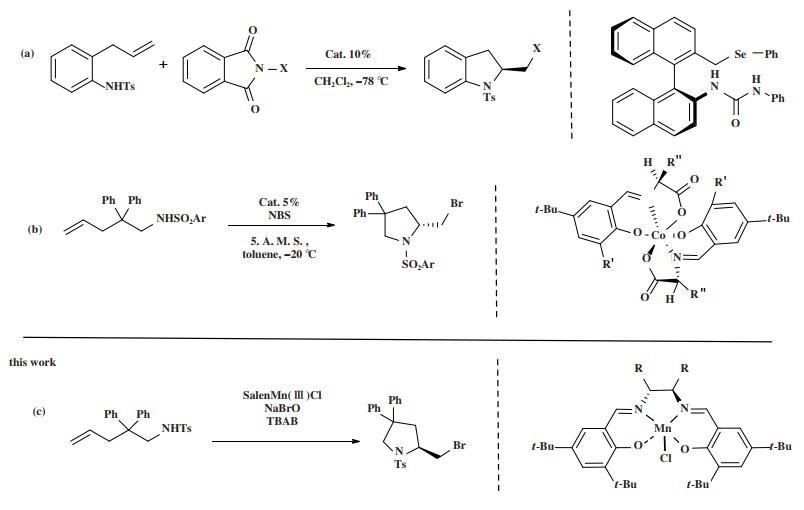
目前对于手性卤代氮杂环化合物的合成方法主要有手性拆分和不对称合成[8-9]等. 手性拆分虽然得到的产品光学纯度高, 但至多只有50%的产率, 易造成原料浪费; 而不对称催化通过使用少量的手性催化剂得到手性产物, 对映选择性好, 产率高, 符合绿色化学的要求, 是合成手性化合物最高效的途径[10-12]. 通过不对称催化合成手性卤代氮杂环已经有许多报道, Okuno团队报道过手性有机硒催化剂催化的烯烃分子内不对称溴胺化反应[13], NBS作为溴源, 最高能够获得92%的ee值(图 1(a)), 具有良好的反应结果. 龚流柱团队报道过钴催化的不对称溴胺化反应[14], 反应产率良好, ee值最高可达到99% (图 1(b)), 手性金属配合物结构整齐, 合成步骤较少, 但催化剂常使用贵金属, 造价高昂. 与其他金属相比, 锰在地壳中含量较多, 性质稳定, 在不对称催化中有良好的应用, 我们之前使用金属锰配合物实现过非手性卤代吡咯烷的合成[15], 也通过锰配合物合成了手性卤代吡咯烷[16], 在此基础上, 进一步改造了催化剂, 在部分产物上得到了更优异的产率和ee值, 并且获得了全新的产物.
|
图 1 不对称卤胺化反应 Fig.1 Asymmetric haloamination reaction |
反应所用烯烃、腈类均购自天津希恩斯生化科技有限公司, 氯苯等溶剂均购自天津市大茂化学试剂厂, 薄层层析和柱层析硅胶(粒径0.050~0.038 mm)购自山东青岛海洋化工有限公司. 所用试剂均为分析纯, 未经纯化直接使用. 戊烯胺和烯丙基苯胺底物均按照文献方法合成[17-18], 催化剂按照已知文献方法制备[19].
Bruker DRX 500核磁共振仪(NMR, 以CDCl3为溶剂, 德国Bruker公司), 化学位移以TMS为内标. X-5显微熔点测试仪(北京泰克仪器责任有限公司), WZZ-2S自动旋光仪(上海仪电光学仪器有限公司), TENSOR红外光谱仪(德国Bruker公司), LabAlliance高效液相色谱仪(HPLC, 天津兰博实验仪器设备有限公司), 手性柱CHIRALPAK IB N-5(大赛璐药物有限责任公司).
1.2 实验过程 1.2.1 手性催化剂合成步骤将乙二胺底物(0.012 5 mol)和3,5-二叔丁基水杨醛(0.05 mol)置于圆底烧瓶中, 加入甲醇溶解, 室温下搅拌反应24 h. 反应结束后蒸除溶剂, 将得到的固体置于圆底烧瓶中, 水浴加热至75 ℃, 在此温度下缓慢加入乙酸乙酯至固体完全溶解, 趁热转移至烧杯中, 静置结晶. 结晶析出后过滤, 取2 mmol结晶, 2 mmol Mn(OAc)2·4H2O置于四口瓶中, 加入15 mL甲苯, 30 mL乙醇溶解, 回流反应2 h. 2 h后加入10 mmol氯化锂, 并鼓吹空气, 继续回流反应3 h. 反应结束后, 静置冷却至室温, 分别用水和饱和食盐水洗涤, 分离有机相, 用无水氯化钙干燥、过滤, 将滤液减压蒸除溶剂, 得到的固体用10 mL二氯甲烷和10 mL正庚烷溶解, 待固体全部溶解后减压蒸馏, 蒸除二分之一溶剂后快速过滤, 滤饼用少量0 ℃的正庚烷洗涤, 取滤饼烘干得到固体即为所需催化剂.
1.2.2 溴胺化反应步骤10 ℃下在反应器中加入氢氧化钠, 磷酸二氢钠, 8 mL次溴酸钠溶液, 调节pH值为10.5~11后加入0.25 mmol底物, 0.062 5 mmol Cat.3, 0.375 mmol四丁基溴化铵, 10 mL氯苯, 10 ℃下反应4 h. 反应结束后用蒸馏水洗涤, 二氯甲烷萃取, 分离有机相, 减压蒸除溶剂, 残留物通过硅胶柱层析, 以石油醚和乙酸乙酯混合物(V∶V=20∶1)为洗脱剂分离得到目标产物, 产物通过高效液相色谱确定手性, 色谱条件为: n-hexane/i-PrOH=80/20, 柱温35 ℃, 1.0 mL/min, λ=220 nm, 产物的1H NMR、13C NMR、HPLC谱图支持信息如下:
(S)-2-溴甲基-4,4-二苯基-1-苯磺酰基四氢吡咯(2a): 白色固体; [α]20D =23.2 (c = 1 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.71~7.62 (m, 2H), 7.46 (t, J = 7.5 Hz, 1H), 7.36 (dd, J = 8.4, 7.1 Hz, 2H), 7.20 (d, J = 5.6 Hz, 4H), 7.15~6.98 (m, 6H), 4.32 (d, J = 10.2 Hz, 1H), 3.96~3.80 (m, 1H), 3.78~3.62 (m, 2H), 2.89 (t, J = 9.9 Hz, 1H), 2.69 (dd, J = 6.5, 4.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 143.49, 136.03, 131.86, 128.13, 127.71, 127.66, 126.33, 125.81, 125.70, 125.53, 125.25, 58.98, 57.76, 51.25, 41.06, 34.65; IR(KBr): 2 874, 2 361, 1 484, 1 445, 1 348, 1 090, 1 025, 866, 753, 697, 663, 610, 575, 451 cm-1; HPLC: CHIRALPAK IB N-5 column, tmajor=9.3 min, tminor= 11.6 min.
(S)-2-溴甲基-4,4-二苯基-1-对甲苯磺酰基四氢吡咯(2b): 白色固体; [α]20D = 24.4 (c =1 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.54 (d, J = 8.0 Hz, 2H), 7.24~6.97 (m, 12H), 4.34 (d, J = 10.2 Hz, 1H), 3.85~3.87(m, 1H), 3.72 (dd, J = 9.8, 3.4 Hz, 1H), 3.63 (d, J = 10.2 Hz, 1H), 2.86 (t, J = 9.9 Hz, 1H), 2.74~2.61 (m, 2H), 2.33 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 144.45, 143.69, 133.84, 129.79, 128.73, 128.65, 127.43, 126.81, 126.58, 126.52, 126.32, 60.06, 58.80, 52.25, 42.04, 35.78, 21.54; IR(KBr): 2 864, 1 595, 1 481, 1 448, 1 357, 1 300, 1 170, 1 089, 1 023, 860, 821, 775, 747, 703, 665, 647, 604, 584, 544, 524, 503, 460, 434, 410 cm-1; HPLC: CHI-RALPAK IB N-5 column, tmajor=8.2 min, tminor= 10.4 min.
(S)-2-溴甲基-4,4-二苯基-1-对叔丁基苯磺酰基四氢吡咯(2c): 白色固体, M.p. 55~58 ℃; [α]20D =38.5 (c = 1 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.62~7.51 (m, 2H), 7.34 (s, 2H), 7.21 (d, J = 4.3 Hz, 4H), 7.13~7.00 (m, 6H), 4.36 (d, J = 10.2 Hz, 1H), 3.83~3.90(m, 1H), 3.74 (dd, J =9.7, 3.4 Hz, 1H), 3.68 (d, J = 10.2 Hz, 1H), 2.86 (t, J =9.9 Hz, 1H), 2.68 (td, J = 13.2, 12.7, 6.5 Hz, 2H), 1.26 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 143.56, 143.45, 132.87, 127.69, 127.59, 126.28, 125.76, 125.67, 125.52, 125.29, 125.11, 58.88, 57.71, 51.16, 41.08, 34.76, 34.10, 30.03; IR (KBr): 3 853, 3 735, 3 649, 2 360, 2 341, 1 698, 1 653, 1 558, 1 541, 1 507, 1 457, 1 261, 1 088, 801, 668, 468, 419 cm-1; HPLC: CHIRALPAK IB N-5 column, tmajor=6.9 min, tminor= 9.5 min.
(S)-2-溴甲基-4,4-二苯基-1-对氯苯磺酰基四氢吡咯(2d): 白色固体, M.p. 144~146 ℃; [α]20D = 34.4(c = 0.05 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.54~7.45 (m, 2H), 7.23~7.15 (m, 4H), 7.14~6.95 (m, 8H), 4.28 (d, J = 10.5 Hz, 1H), 3.94~3.83 (m, 1H), 3.78 (ddd, J = 24.6, 10.2, 2.2 Hz, 2H), 3.07 (t, J = 9.6 Hz, 1H), 2.86 (ddd, J = 13.3, 7.5, 1.2 Hz, 1H), 2.55 (dd, J = 13.3, 6.4 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 143.58, 142.76, 138.27, 134.56, 128.32, 127.70, 127.4, 125.8, 125.60, 125.38, 125.25, 58.99, 58.34, 51.43, 41.20, 35.02; IR (KBr): 3 103, 2 919, 2 850, 2 360, 1 903, 1 588, 1 497, 1 396, 1 348, 1 124, 1 095, 1 056, 1 015, 926, 885, 780, 769, 708, 574, 507 cm-1; HPLC: CHIRALPAK IB N-5 column, tmajor=9.7 min, tminor= 13.5 min.
(S)-2-溴甲基-4,4-二苯基-1-对溴苯磺酰基四氢吡咯(2e): 白色固体, M.p. 130~132 ℃; [α]20D = 24 (c = 1 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.49 (q, J = 8.8 Hz, 4H), 7.29~7.25 (m, 2H), 7.23~7.10 (m, 6H), 7.06 (dd, J = 7.7, 2.1 Hz, 2H), 4.36 (d, J = 10.5 Hz, 1H), 3.95 (dddd, J = 9.5, 7.4, 6.4, 3.2 Hz, 1H), 3.86 (ddd, J =22.3, 10.2, 2.2 Hz, 2H), 3.15 (t, J = 9.7 Hz, 1H), 3.92~3.98(m, 1H), 2.63 (dd, J = 13.3, 6.4 Hz, 1H); 13C NMR (101 MHz, CDCl3)δ 144.63, 143.80, 136.11, 132.35, 128.77, 128.61, 126.87, 126.68, 126.44, 126.30, 60.08, 59.42, 52.49, 42.30, 36.03; IR(KBr): 3 735, 2 361, 2 341, 1 574, 1 473, 1 392, 1 347, 1 166, 1 090, 1 068, 1 029, 1 010, 827, 759, 735, 701, 668, 609, 577, 556, 507, 485, 467, 446, 421 cm-1; HPLC: CHIRALPAK IB N-5 column, tmajor=10.9 min, tminor=16.8 min.
(S)-2-溴甲基-4,4-二苯基-1-(3-氯-苯磺酰基)-四氢吡咯(2f): 白色固体; [α]20D = 11.68 (c = 0.5 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.66 (t, J = 1.9 Hz, 1H), 7.57 (dt, J = 7.8, 1.4 Hz, 1H), 7.49~7.42 (m, 1H), 7.36~7.26 (m, 3H), 7.24~7.05 (m, 8H), 4.34 (d, J =10.5 Hz, 1H), 4.01 (dq, J = 7.5, 2.0, 1.0 Hz, 1H), 3.94 (dd, J = 10.4, 1.3 Hz, 1H), 3.82 (dd, J = 9.9, 3.2 Hz, 1H), 3.16 (t, J = 9.6 Hz, 1H), 2.95 (ddd, J = 13.3, 7.5, 1.3 Hz, 1H), 2.68 (dd, J = 13.3, 6.4 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 143.48, 142.66, 138.12, 134.31, 131.96, 129.28, 127.73, 127.65, 126.24, 125.91, 125.85, 125.41, 125.19, 124.27, 58.95, 58.18, 51.45, 41.25, 34.89; IR(KBr): 3 734, 3 648, 2 923, 2 360, 1 541, 1 457, 1 349, 1 165, 1 081, 1 030, 795, 749, 701, 669, 608, 576, 423 cm-1; HPLC: CHIRALPAK IB N-5 column, tmajor=9.5 min, tminor=13.9 min.
(S)-2-溴甲基-4,4-二苯基-1-萘磺酰基四氢吡咯(2g): 白色固体; [α]20D =11.6 (c =0.01 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 8.30 (d, J = 1.8 Hz, 1H), 7.90 (dd, J = 20.5, 8.0 Hz, 2H), 7.82 (d, J = 8.7 Hz, 1H), 7.65 (tt, J = 9.8, 1.8 Hz, 2H), 7.33~7.15 (m, 6H), 7.05~7.00 (m, 2H), 6.95 (t, J = 7.6 Hz, 2H), 6.86 (t, J = 7.2 Hz, 1H), 4.43 (d, J = 10.3 Hz, 1H), 4.11~4.01 (m, 1H), 3.93~3.82 (m, 2H), 3.07 (t, J = 9.8 Hz, 1H), 2.85 (dd, J = 13.2, 7.7 Hz, 1H), 2.68 (dd, J = 13.2, 5.7 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 143.94, 132.14, 129.42, 129.38, 128.85, 128.72, 128.69, 128.46, 127.85, 127.46, 126.81, 126.52, 126.48, 126.16, 122.55, 60.09, 59.06, 52.39, 42.19, 35.99; IR(KBr): 3 738, 2 361, 2 334, 1 740, 1 499, 1 340, 1 161, 1 078, 1 027, 819, 753, 697, 664, 548, 464 cm-1; HPLC: CHIRALPAK IB N-5 column, tmajor=10.9 min, tminor=14.4 min.
(S)-2-溴甲基-4,4-二苯基-1-甲磺酰基四氢吡咯(2h): 白色固体; [α]20D = 85 (c = 1 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.33 (d, J = 6.9 Hz, 2H), 7.30~7.20 (m, 4H), 7.18 ~7.10 (m, 4H), 4.23 (dd, J = 10.9, 1.9 Hz, 1H), 4.09 (d, J = 11.0 Hz, 1H), 4.04~3.89 (m, 1H), 3.68 (dd, J = 10.1, 2.9 Hz, 1H), 3.35 (dd, J = 10.1, 8.2 Hz, 1H), 3.16 (ddd, J = 13.4, 6.9, 1.9 Hz, 1H), 2.58~2.49 (m, 1H), 2.30 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 143.54, 143.06, 127.97, 127.77, 126.19, 125.89, 125.80, 125.41, 58.82, 58.44, 52.20, 41.64, 35.64, 35.56; HPLC: CHIRALPAK AD-H, hexane/ethanol/ethylenediamine = 70/30/0.1, 1.0 mL/min, λ = 254 nm, tmajor=9.8 min, tminor= 16.6 min.
(S)-2-溴甲基-4,4-二甲基-1-对甲苯磺酰基四氢吡咯(2i): 白色固体; [α]20D = 22.4 (c = 0.05 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.67 (d, J = 8.0 Hz, 2H), 7.25 (s, 2H), 3.87 (dd, J = 9.7, 3.0 Hz, 1H), 3.79 (qd, J = 8.2, 3.0 Hz, 1H), 3.45 (t, J = 9.2 Hz, 1H), 3.17~2.98 (m, 2H), 2.37 (s, 3H), 1.86~1.74 (m, 1H), 1.63 (dd, J = 12.9, 8.3 Hz, 1H), 0.98 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 142.70, 133.73, 128.69, 126.48, 60.83, 58.98, 44.82, 36.49, 36.46, 25.05, 24.73, 20.55; IR(KBr): 3 438, 2 922, 2 357, 1 597, 1 494, 1 451, 1 342, 1 189, 1 156, 1 111, 1 090, 1 032, 1 001, 920, 804, 742, 700, 664, 603, 570, 545, 506, 474, 426 cm-1; HPLC: CHIRALPAK IB N-5 column, tmajor=6.0 min, tminor= 6.4 min
(S)-2-溴甲基-1-对甲苯磺酰基四氢吡咯(2j): 油状液体; [α]20D = 38.4 (c = 0.01 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.78~7.69 (m, 2H), 7.34 (d, J = 8.0 Hz, 2H), 3.86~3.79 (m, 1H), 3.79~3.73 (m, 1H), 3.47 (ddd, J = 10.0, 6.6, 4.7 Hz, 1H), 3.36 (t, J = 9.7 Hz, 1H), 3.15 (ddd, J = 10.0, 7.7, 6.7 Hz, 1H), 2.44 (s, 3H), 2.00~1.89 (m, 1H), 1.89~1.79 (m, 1H), 1.79~1.68 (m, 1H), 1.55 (dtt, J = 11.3, 6.4, 4.5 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 143.83, 133.98, 129.85, 127.54, 60.38, 49.83, 36.08, 30.26, 23.81, 21.58; IR(KBr): 3 828, 3 728, 3 623, 2 964, 2 924, 2 861, 2 361, 2 334, 1 741, 1 596, 1 497, 1 459, 1 340, 1 260, 1 200, 1 095, 1 027, 983, 925, 872, 810, 753, 666, 584, 548, 463 cm-1 HPLC: CHIRALPAK IB N-5 column, tmajor=7.2 min, tminor= 7.9 min.
(S)-3-溴甲基-2-对甲苯磺酰基-2-氮杂螺[4, 4]-壬烷(2k): 黄色固体; M.p. 72~75 ℃; [α]20D = 12.9 (c = 0.02 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 8.3 Hz, 2H), 7.34 (d, J = 8.0 Hz, 2H), 3.95 (dd, J = 9.7, 3.2 Hz, 1H), 3.76~3.83(m, 1H), 3.49 (t, J = 9.6 Hz, 1H), 3.29 (d, J = 10.3 Hz, 1H), 3.11 (d, J = 10.3 Hz, 1H), 2.44 (s, 3H), 1.94 (ddd, J = 12.9, 7.5, 1.2 Hz, 1H), 1.83 (dd, J = 12.9, 7.1 Hz, 1H), 1.65~1.38 (m, 6H), 1.01 (ddd, J = 12.6, 8.0, 6.7 Hz, 1H), 0.88 (ddd, J =12.9, 7.9, 5.8 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 143.80, 134.39, 129.75, 127.58, 60.36, 48.57, 43.51, 37.03, 36.50, 36.25, 24.50, 24.29, 21.60; IR(KBr): 2 952, 2 360, 1 344, 1 161, 1 037, 664, 637, 599, 574, 550, 499, 465, 441, 425 cm-1; HPLC: CHIRALPAK IB N-5 column, tmajor=5.6 min, tminor= 5.9 min.
(S)-2-溴丙基-1-对甲苯磺酰基四氢吡咯(2l): 白色固体; M.p. 132~134 ℃; [α]20D = 12.5 (c = 0.1 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 8.3 Hz, 2H), 7.31~7.25 (m, 2H), 7.19~7.12 (m, 7H), 6.97 (dd, J = 7.3, 2.4 Hz, 3H), 4.64 (t, J = 7.3 Hz, 1H), 3.79 (t, J = 6.3 Hz, 1H), 3.42 (d, J = 6.3 Hz, 2H), 2.72 (d, J =7.2 Hz, 2H), 2.33 (s, 3H), 1.47 (d, J = 1.4 Hz, 3H), 1.30 (d, J = 1.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 143.68, 134.49, 128.60, 127.44, 127.25, 126.86, 126.51, 126.09, 125.56, 125.49, 117.40, 49.07, 48.63, 34.46, 24.91, 20.49, 16.84; IR(KBr): 3 438, 2 922, 1 597, 1 494, 1 451, 1 342, 1 156, 1 090, 1 032, 805, 742, 700, 664, 603, 570, 545, 474, 426 cm-1; HPLC: CHIRALPAK IB N-5 column, tmajor=6.7 min, tminor=7.4 min.
(S)-2-溴甲基-1-对甲苯磺酰基-5-叔丁基-2, 3-二氢吲哚(2u): 白色固体; 1H NMR (400 MHz, CDCl3) δ 7.55 (dd, J = 13.7, 8.4 Hz, 3H), 7.27~7.15 (m, 4H), 7.07 (d, J = 1.4 Hz, 1H), 4.37~4.43(m, 1H), 3.83 (dd, J = 9.8, 3.8 Hz, 1H), 3.39 (t, J = 10.0 Hz, 1H), 2.98~2.81 (m, 2H), 2.37 (s, 3H), 1.27 (s, 9H).; 13C NMR (101 MHz, CDCl3) δ 148.21, 144.14, 138.5, 134.57, 130.14, 129.72, 127.12, 124.97, 122.22, 116.18, 62.37, 35.87, 34.49, 33.37, 31.48, 21.59; IR(KBr): 3 737, 2 955, 2 361, 2 334, 1 596, 1 489, 1 352, 1 305, 1 215, 1 121, 1 091, 1 035, 968, 890, 821, 755, 705, 668, 586, 547, 430 cm-1; HPLC: CHIRALPAK IB N-5 column, tmajor=5.6 min, tminor= 5.9 min.
(S)-2-氯甲基-4,4-二苯基-1-苯磺酰基四氢吡咯(3a): 白色固体, M.p. 131~133 ℃; [α]20D = 70 (c =0.5 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 7.1 Hz, 2H), 7.54 (t, J = 7.5 Hz, 1H), 7.43 (t, J = 7.7 Hz, 2H), 7.28 (d, J = 5.2 Hz, 4H), 7.20~7.07 (m, 6H), 4.37 (d, J = 10.2 Hz, 1H), 3.98~3.84 (m, 2H), 3.75 (d, J = 10.2 Hz, 1H), 3.16~3.04 (m, 1H), 2.74 (dd, J = 6.4, 3.4 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 144.57, 136.95, 132.94, 129.19, 128.76, 128.71, 127.40, 126.85, 126.76, 126.58, 126.31, 60.18, 58.61, 52.32, 46.44, 41.01; IR(KBr): 3 069, 3 002, 2 956, 2 920, 2 850, 2 361, 1 597, 1 481, 1 354, 1 253, 1 172, 1 092, 1 049, 1 020, 997, 895, 827, 777, 750, 736, 704, 692, 610, 588, 573, 546, 526 cm-1; HPLC: CHIRALPAK IB N-5 column, tmajor = 8.6 min, tminor = 10.6 min.
(S)-2-氯甲基-4,4-二苯基-1-对甲苯磺酰基四氢吡咯(3b): 白色固体; [α]20D = 45.2 (c = 0.5 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.64~7.43 (m, 2H), 7.19 (d, J = 4.3 Hz, 4H), 7.14~6.97 (m, 8H), 4.31 (d, J = 10.2 Hz, 1H), 3.88~3.72 (m, 2H), 3.60 (d, J = 10.2 Hz, 1H), 2.99 (t, J = 10.9 Hz, 1H), 2.65 (d, J = 6.2 Hz, 2H), 2.30 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 143.61, 132.67, 128.74, 127.66, 127.59, 126.37, 125.72, 125.51, 125.46, 125.26, 59.13, 57.56, 51.23, 45.49, 39.90, 20.49; IR(KBr): 2 959, 2 866, 2 360, 2 335, 1 869, 1 684, 1 596, 1 490, 1 449, 1 358, 1 261, 1 171, 1 089, 1 025, 821, 748, 703, 662, 606, 587, 542 cm-1; HPLC: CHIRALPAK IB N-5 column, tmajor= 8.6 min, tminor= 10.8 min.
(S)-2-氯甲基-1-对甲苯磺酰基四氢吡咯(3j): 油状液体; [α]20D = 0.4 (c = 1 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 6.4 Hz, 2H), 7.34 (d, J = 8.0 Hz, 2H), 3.88 (dd, J = 10.6, 3.4 Hz, 1H), 3.84~3.73 (m, 1H), 3.55~3.40 (m, 2H), 3.13 (dd, J = 16.8, 7.9 Hz, 1H), 2.44 (s, 3H), 1.94 (dq, J = 12.0, 3.9, 3.2 Hz, 1H), 1.84 (dt, J = 12.2, 7.6 Hz, 1H), 1.76~1.64 (m, 1H), 1.64~1.50 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 143.83, 133.92, 129.84, 127.54, 60.51, 49.67, 47.02, 29.27, 23.80, 21.57; IR(KBr): 2 362, 2 336, 1 868, 1 845, 1 734, 1 717, 1 684, 1 636, 1 559, 1 541, 1 521, 1 507, 1 457, 1 419, 1 339, 1 199, 1 160, 1 092, 1 038, 989, 831, 815, 766, 729, 667, 587, 550 cm-1; HPLC: CHIRALPAK IB N-5 column, tmajor=7.3 min, tminor= 7.9 min.
(S)-2-氯甲基-1-对甲苯磺酰基-2,3-二氢吲哚(3v) : 白色固体; [α]20D = 38.5 (c = 1 in CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.66 (d, J = 8.1 Hz, 1H), 7.60~7.52 (m, 2H), 7.20 (dd, J = 10.7, 8.1 Hz, 3H), 7.10~7.01 (m, 2H), 4.41 (ddd, J = 9.2, 5.2, 3.8 Hz, 1H), 3.93 (dd, J = 10.7, 3.9 Hz, 1H), 3.55 (dd, J = 10.7, 9.6 Hz, 1H), 2.99~2.82 (m, 2H), 2.36 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 144.29, 134.47, 129.75, 127.97, 127.06, 125.27, 124.98, 116.92, 62.30, 46.91, 32.23, 21.57; IR(KBr): 3 286, 2 919, 2 360, 1 917, 1 844, 1 749, 1 645, 1 597, 1 580, 1 491, 1 457, 1 397, 1 331, 1 305, 1 232, 1 158, 1 090, 1 044, 993, 919, 814, 767, 669, 568, 532 cm-1; HPLC: CHIRALPAK IB N-5 column, tmajor=5.5 min, tminor= 6.0 min.
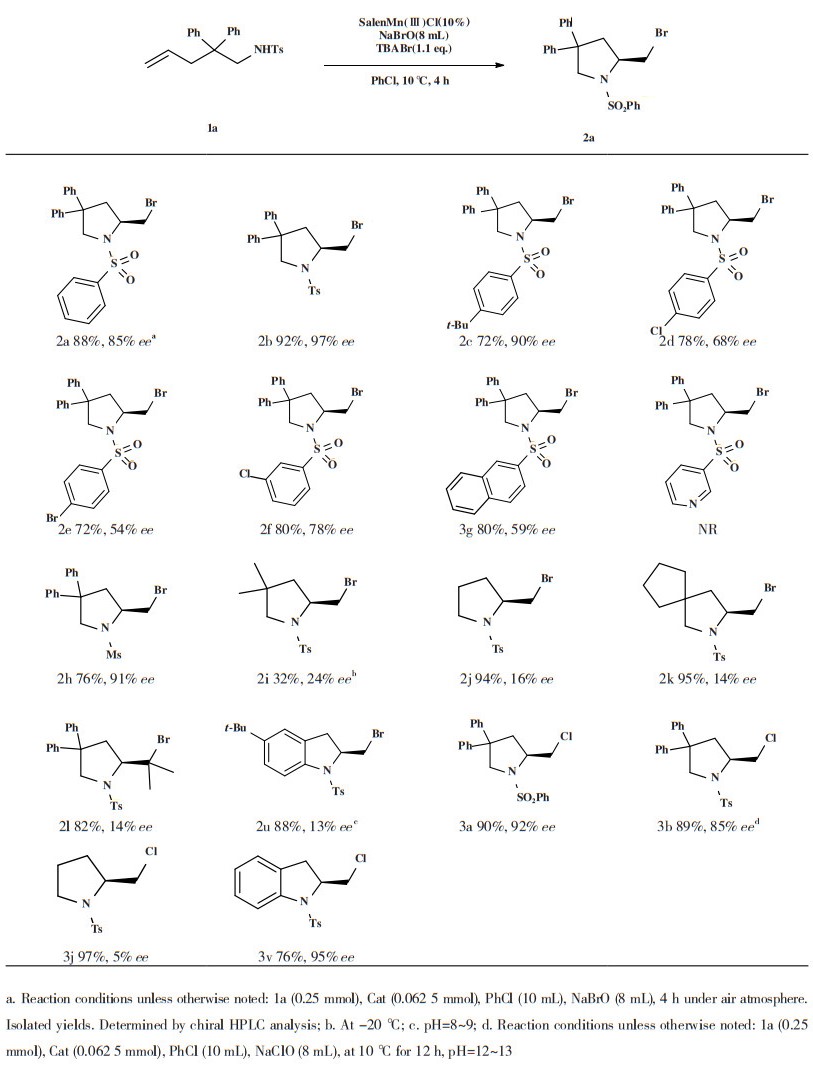
2 结果与讨论 2.1 反应条件对催化作用的影响首先使用1a作为底物, NaBrO作为溴源, 二氯甲烷作为溶剂, 室温下进行反应, 选取手性席夫碱配体与金属锰配合生成手性催化剂进行反应, 得到溴代吡咯烷产物, 在此基础上探究反应条件对结果的影响, 结果如表 1所示.
| 表 1 反应条件优化a Table 1 Optimization of reaction conditionsa |
配体3位上取代基的空间位阻对于催化剂的作用有着重要影响[20], 考察了带有不同取代基的催化剂对反应对映选择性的影响(表 1, entry 1-3), 结果发现连接位阻较大的取代基对反应对映选择性有积极影响, 其中Cat.3得到最佳的ee值(26%). 考察了常用溶剂对反应的影响(表 1, entry 4-6), 在实验的多种溶剂中, 常用的非质子性溶剂能够以较高的产率得到溴胺化产物, 但对映选择性较差, 苯系溶剂表现出更好的对映选择性, 其中氯苯获得了最佳的结果, 得到36%的ee值. 温度对于反应的对映选择性存在影响, 通常低温条件下反应有利于对映选择性的提升[21-23], 因此考察了不同温度对于反应结果的影响(表 1, entry 7-10), 结果在ee值改变不大的情况下, 0 ℃下的反应产率降低, 由于反应体系中有水的存在, 低温会影响反应产率, 反应温度在0 ℃以上会更有利, 进一步控制温度在10 ℃时获得了最佳的ee值(45%). 考察了催化剂用量对反应的影响(表 1, 11-12), 结果显示当用量从5%上升至10%(摩尔分数)时, ee值上升到84%, 进一步增加催化剂的量到20%(摩尔分数)时, 产物的ee值下降, 基于对映选择性和成本, 选定最佳反应条件是氯苯作为溶剂, 催化剂使用Cat. 3添加量为10%(摩尔分数), 10 ℃下反应4 h.
2.2 底物适用性研究确定最优反应条件后, 在最优条件下探究了该反应条件对底物的适用性, 如表 2所示. 当底物的苯磺酰基苯环对位连有给电子基甲基、叔丁基时, 产率在70%以上, ee值在90%以上; 对位连弱吸电子基氯或溴时, ee值保持中等水平, 分别为67%和53%. 当卤素基团连接在间位时, 得到78%的ee值. 当连有吡啶磺酰基时并未得到目标产物, 连有萘磺酰基时得到59%的ee值, 连有甲基磺酰基时得到91%的ee值. 之后探究了底物的直链部分取代基对反应结果的影响, 将4位的苯基替换为空间位阻更小的甲基和氢原子时, ee值分别为25%和14%. 4位连接环戊基时, ee值为16%, 底物4位上取代基的位阻对反应的产率影响较小. 之后用烯丙基苯胺类底物进行反应, 以88%的产率得到溴代吲哚产物, ee值为13%.
| 表 2 底物扩展 Table 2 Substrate scope |
此外, 对卤源进行扩展, 采用NaClO作为卤源进行氯胺化反应, 结果与溴胺化反应具有相似的结果, 能以较高的产率和对映选择性得到氯代吡咯烷产物, 在4位取代基位阻较小的情况下能以97%的产率得到氯代产物, 但产物对映选择性较低. 当使用烯丙基苯胺类底物进行反应时, 在以较高的产率得到吲哚产物的同时, 产物的对映选择性良好, ee值为95%.
2.3 构建手性中心的催化机理基于相关文献和实验结果[24-25], 提出一个机理, 如图 2所示. 底物在NaBrO的作用下得到卤素与氮相连的中间体A, A与SalenMn(Ⅲ)结合得到一个含氮锰双键的中间态, 其双键裂解得到自由基中间体B, 中间体B与烯烃发生单一方向的分子内自由基加成, 生成手性环状中间体C, 在这一步骤中, 底物中的磺酰基与锰配合物中的芳环存在空间排斥, 使得N自由基进攻双键具有方向性, 导致产物具有手性. 由于四元环的不稳定性, 锰配合物离去, 四元环开环得到氮杂环丙烷并吡咯烷中间体D, 溴负离子亲核进攻化合物C上带正电荷的N, 开环得到溴代吡咯烷产物.
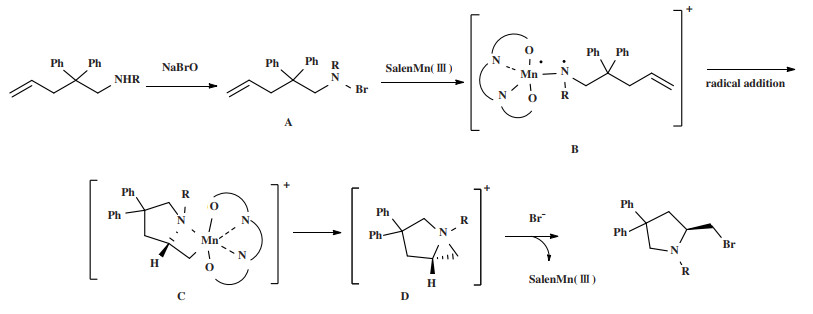
|
图 2 反应机理推测 Fig.2 Possible mechanism |
将手性SalenMn(Ⅲ)催化剂用于非活化烯烃的分子内不对称卤胺化反应, 在次卤酸钠的存在下, 得到了一系列手性卤代吡咯烷产物, 筛选出最佳催化剂Cat.3, 用量为10%(摩尔分数), 将该催化剂用于不同底物进行反应, 以较好的产率和对映选择性得到邻位卤代吡咯烷产物, 反应条件温和, ee值最高达到97%, 提出了一个反应机理, 反应通过氮自由基对双键的方向性进攻构建手性中心.
| [1] |
Philipp K, Frank P, Silvio R, et al. Chirality in DrugResearch[M]. Germany: Wiley-VCH Verlag GmbH, 2006.
|
| [2] |
Huang Xian-qi (黄贤琦). Clemastine fumarate tablets (富马酸氯马斯汀片)[J]. Chin New Drugs J(中国新药杂志), 1998, 7(3): 194-195.
|
| [3] |
Fan Xin-xing (樊新星), Xu Ting (徐珽), Lu Jing (卢静), et al. Vildagliptin: A new agent for diabetic treatment(抗糖尿病新药维格列汀)[J]. Chin New Drugs J(中国新药杂志), 2008, 17(14): 1272-1274.
|
| [4] |
Nakamura T, Okuno K, Kaneko K, et al. Chiral bifunctional sulfide-catalyzed asymmetric bromoaminocyclizations[J]. Org Biomol Chem, 2020, 18(17): 3367–3373.
DOI:10.1039/D0OB00459F |
| [5] |
Jiang X J, Liu S H, Yang S, et al. Enantioselective bromolactonization of deactivated olefinic acids[J]. Org Lett, 2018, 20(11): 3259–3262.
DOI:10.1021/acs.orglett.8b01125 |
| [6] |
Wang H, Frings M, Bolm C. Halocyclizations of unsaturated sulfoximines[J]. Org Lett, 2016, 18(10): 2431–2434.
DOI:10.1021/acs.orglett.6b00958 |
| [7] |
Qiu Xian-hua(邱献华), Wang Mei-jun(王美君), Zhang Mei-hui(张美慧), et al. Graphical synthetic routes to larotrectinib(拉罗替尼合成路线图解)[J]. Chin J Med Chem(中国药物化学杂志), 2019, 29(4): 325-328.
|
| [8] |
Reynolds M, Eary C T, Spencer S, et al. Preparation of (S)-N-(5-((R)-2-(2,5-difluorophenyl) pyrrolid-in-1-yl) Pyrazolo[1, 5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide[P]. EP, 3800189A, 2017.18.05
|
| [9] |
Wang Y Q, Zheng J L, Zhan Y J. A Synthesis Method of (R)-3-hydroxypyrrolidine[P]. CN, 112661687A, 2021.04.16.
|
| [10] |
Noyori R. Asymmetric catalysis: Science and opportunities[J]. Angew Chem, 2002, 41(12): 2008–2022.
DOI:10.1002/1521-3773(20020617)41:12<2008::AID-ANIE2008>3.0.CO;2-4 |
| [11] |
Sandeep K S, Alilugatta S R P, Yashwant B T. Developments in the catalytic asymmetric synthesis of agrochemicals and their synthetic importance[J]. J Agric Food Chem, 2021, 69(49): 14761–14780.
DOI:10.1021/acs.jafc.1c05553 |
| [12] |
Zhang Y Y, Yao W, Li H, et al. Rare-earth-metal complexes supported by new chiral tetra-azane chelating ligands: Synthesis, characterization and catalytic properties for intramolecular asymmetric hydroamination[J]. Organometallics, 2012, 31(13): 4670–4679.
DOI:10.1021/om201286u |
| [13] |
Okuno K, Nakamura T, Shirakawa S. Asymmetric catalysis of chiral bifunctional selenides and selenonium salts bearing a urea group[J]. Asian J Org Chem, 2021, 10(3): 3406–3410.
|
| [14] |
Jiang H J, Liu K, Gong L Z. Switchable stereoselectivity in bromoaminocyclization of olefins catalyzed by Brönsted acids of anionic chiral Co(Ⅲ) complexes[J]. Angew Chem, Int Ed Engl, 2017, 56(39): 11931–11935.
DOI:10.1002/anie.201705066 |
| [15] |
Sun H, Cui B, Liu G S, et al. MnI2-Catalyzed regioselective intramolecular iodoamination of unfunctionalized olefins[J]. Tetrahedron, 2016, 72(45): 7170–7178.
DOI:10.1016/j.tet.2016.09.038 |
| [16] |
Sun H, Shang H J, cui B. (Salen)Mn(Ⅲ)-catalyzed enantioselective intramolecular haloamination of alkenes through chiral aziridinium ion ring-opening sequenc[J]. ACS Catal, 2022, 12(12): 7046–7053.
DOI:10.1021/acscatal.2c02223 |
| [17] |
Shigehisa H, Koseki N, Shimizu N, et al. Catalytic hydroamination of unactivated olefins using a Co catalyst forcomplex molecule synthesis[J]. J Am Chem Soc, 2014, 136(39): 13534–13537.
DOI:10.1021/ja507295u |
| [18] |
Muñiz K, Hövelmann C H, Streuff J. Oxidative diamination of alkenes with ureas as nitrogen sources: Mechanistic pathways in the presence of a high oxidation state palladium catalyst[J]. J Am Chem Soc, 2008, 130(2): 763–773.
DOI:10.1021/ja075041a |
| [19] |
Wang Ji-tao(王积涛), Chen Rong(陈蓉), Feng Xiao(冯霄), et. al. Studies of asymmtric epoxidation of styrenecatalyzed by chiral metal-salen complexes(手性过渡金属(Mn、Co、Ni)-Salen配合物催化NaOCl不对称环氧化苯乙烯的反应研究)[J]. Chin J Org Chem(有机化学), 1998, 18(3): 228-234.
|
| [20] |
Srinivasan K, Michaud P, Kochi J K. Epoxidation of olefinswith cationic (salen)manganese(Ⅲ) complexes. The modulation of catalytic activity by substituents[J]. J Am Chem Soc, 1986, 108(9): 2309–2320.
DOI:10.1021/ja00269a029 |
| [21] |
Mu Hong-wen(穆宏文), Du Nan(杜楠), Jin Ying(金瑛), et al. Organocatalyzed enantioselective aldol reaction of isatins and acetoacetates(脲衍生物有机催化靛红与乙酰乙酸酯的不对称Aldol反应)[J]. J Mol Catal(China)(分子催化), 2022, 36(1): 51-57.
|
| [22] |
Ge Wei-wei(葛伟伟), Kong Fan-hua(孔凡华), HuangLi-hua(黄力华). Asymmetric synthesis of α - arylpropanolcompounds by chiral phosphoramide compounds catalysts(手性磷酰胺类化合物不对称催化合成α-芳基丙醇类化合物)[J]. J Mol Catal(China)(分子催化), 2022, 36(1): 22-31.
|
| [23] |
Nie J, Hua M Q, Xiong H Y, et al. Asymmetric phase-transfer-catalyzed conjugate addition of glycine imine to exocyclic α, β-unsaturated ketones: Construction of polycyclic imines containing three stereocenters[J]. J Org Chem, 2012, 77(9): 4209–4216.
DOI:10.1021/jo300500r |
| [24] |
Ju M, Jennifer M S. Nitrene transfer catalysts for enantioselective C-N bond formation[J]. Nat Rev Chem, 2021, 5(8): 580–594.
DOI:10.1038/s41570-021-00291-4 |
| [25] |
Snider B B, Duvall J R. Termination of Mn(Ⅲ)-based oxidative cyclizations by trapping with azide[J]. Org Lett, 2004, 6(8): 1265–1268.
DOI:10.1021/ol049805s |
 2023, Vol. 37
2023, Vol. 37